Goats As Sentinel Hosts for the Detection of Tick-Borne Encephalitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Streiflichter Zur Hotel- Und Tourismusgeschichte Im Saastal Bis Zum Ersten Weltkrieg 1
STREIFLICHTER ZUR HOTEL- UND TOURISMUSGESCHICHTE IM SAASTAL BIS ZUM ERSTEN WELTKRIEG 1 Roland Flückiger-Seiler 1 Die Entdeckung und Erforschung der Alpenwelt Wichtige Grundlagen für die Entdeckung der Schweizer Alpen als «Garten Edens» leisteten engagierte Forscher und Schriftsteller bereits im frühen 18. Jahrhun- dert. Einer der Ersten war der Zürcher Arzt und Universalgelehrte Johann Jakob Scheuchzer (1672–1733), der sich als erster Naturforscher nach dem lähmenden 17. Jahrhundert mit wissenschaftlichen Absichten in die Alpen wagte. In den Som- mermonaten durchstreifte er mit seinen Studenten alpine Gegenden und sammelte alle erdenklichen Daten über Natur und Umwelt. Mit diesem Wissen publizierte er 1713 eine vierteilige Karte der Schweiz, die lange Zeit als beste Darstellung der Eidgenossenschaft galt. Die von ihm erhobenen Daten und die eigene Beobach- tung des Gebirges auf seinen Wanderungen über fast alle Schweizer Alpenpässe bildeten die Basis für das seit 1702 in mehreren Auflagen erschienene Lebenswerk ‹Itinera per Helvetiae alpinas regiones›. Bereits 1704 nahm ihn die «Royal Socie- ty» in London als Mitglied auf, eine damals äusserst seltene Ehre für einen Aus- länder. Die Verbreitung seiner naturwissenschaftlichen Arbeiten über die Schweiz 1 Diese Übersicht basiert auf Texten, die der Autor in seinem Buch ‹Berghotels zwischen Alp- weide und Gipfelkreuz› 2015 im Verlag Hier und Jetzt, Baden, veröffentlicht hat. Inzwischen sind noch weitere Fakten zum Vorschein gekommen und die Texte zum Saastal sind hier über- sichtlich zusammengefasst. Der Autor möchte bei dieser Gelegenheit allen danken, die ihn bei seinen Forschungen im Saastal unterstützt haben und noch unterstützen: In erster Linie Damian Bumann als Archivar im «Historischen Archiv Saastal» (HAS). Dann geht der Dank auch an den Stiftungsrat von «Saas ischi Heimat» sowie an Beat Anthamatten und Bernhard Andenmatten, die mir zu wertvollem Material verholfen haben. -

News Aus Dem Gemeinderat
News aus dem Gemeinderat Sitzung vom Dienstag, 27. Oktober 2020 Baubewilligungen 20121 Aldo und Nadja Mathieu Neubau EFH vorzeitiger Baubeginn Parzelle Nr. 7874, Suste, Susten 20140 Flavian Kippel Ersetzen von best. Bauplakat Parzelle Nr. 7677, Oberbann, Leuk-Stadt 20118 Schweiz. Bundesbahnen SBB Bern Dienstgebäude DG01, Anpassung Fassade Stefan Chanton Betriebsbewilligung Hotel / Restaurant "Links Leuk Golfresort", Susten Der Gemeinderat beschliesst die Genehmigung folgender Betriebsbewilligung, nach erfolgter öffentlicher Publikation, ohne Einsprache. Gesuchsteller: Stefan Chanton Betrieb: Hotel / Restaurant « Link Leuk Golfresort » Dienstleistungen: Gewerbsmässiges Angebot von Beherbergung und von Speisen, alkoholischen oder alkoholfreien Getränken zum Genuss vor Ort. Öffnungszeiten: Montag – Sonntag 08.00 – 23.00 Uhr Beginn: sofort Gebäude Einkauf Pellets, Vergabe Lieferauftrag Für die Gemeindegebäude mit Pelletsfeuerung (Sportplatz, Werkhof/FW Lokal, DiLEi) ist der Einkauf von Pellets vorzunehmen. Der Gemeinderat beschliesst, 1/3 der Pelletsmenge bei Valais Pellets, Ernen zum Preis von CHF 10'350.- und 2/3 bei Matterhorn Pellet AG, Zermatt zum Preis von CHF 18'000.- Total CHF 28'350.- einzukaufen. Interkommunaler Grundsatzentscheid, Projektleitung Naturpark Pfyn Finges Richtplan An der Präsidentenkonferenz des Bezirks Leuk am 19.08.2020 wurde das Projekt eines Interkommunalen Richtplans präsentiert. Es geht um die Abstimmung von kommunalen Zonennutzungsplanungen mit regionalen Vorgaben seitens des Kantons, so z.B. in Sachen touristischer -

Lithostratigraphy and U-Pb Zircon Dating in the Overturned Limb of the Siviez-Mischabel Nappe: a New Key for Middle Penninic Nappe Geometry
1661-8726/08/020431-22 Swiss J. Geosci. 101 (2008) 431–452 DOI 10.1007/s00015-008-1261-5 Birkhäuser Verlag, Basel, 2008 Lithostratigraphy and U-Pb zircon dating in the overturned limb of the Siviez-Mischabel nappe: a new key for Middle Penninic nappe geometry FLORIAN GENIER1, JEAN-LUC EPARD 1, FRANÇOIS BUSSY 2 & TOMAS MAGNA2 Key words: alps, Middle Penninic, Siviez-Mischabel nappe, Permo-Carboniferous, Randa orthogneiss, zircon typology, U-Pb geochronology ABSTRACT Detailed field work and zircon analysis have improved the knowledge of the This coherent overturned sequence can be observed from the St-Niklaus area to lithostratigraphy at the base of the Siviez-Mischabel nappe in the Mattertal the Moosalp pass to the north. Detailed mapping revealed that the St-Niklaus (St-Niklaus-Törbel area). They confirm the existence of an overturned limb syncline is symmetrical and connects the overturned limb of the Siviez-Mischa- and clarify the structure of the St-Niklaus syncline. The following formations bel nappe to the normal series of the Upper Stalden zone. U-Pb zircon geo- can be observed: chronology on magmatic and detrital zircons allowed constraining ages of these formations. Detrital zircons display ages ranging from 2900 ± 50 to 520 ± 4 Ma • Polymetamorphic gneisses; composed of paragneisses, amphibolites and in the Törbel Formation, and from 514 ± 6 to 292 ± 9 Ma in the Moosalp Forma- micaschists (Bielen Unit, pre-Ordovician). tion. In addition, the Permian Randa orthogneiss is intrusive into the polymeta- • Fine-grained, greyish quartzite and graywacke with kerogen-rich hori- morphic gneisses and into the Permo-Carboniferous metasediments of the zons (Törbel Formation, presumed Carboniferous). -

Price-Martin-F ... Rockies and Swiss Alps.Pdf
Price, Martin Francis (Ph.D., Geography) Mountain forests as common-property resources: management policies and their outcomes in the Colorado Rockies and the Swiss Alps. Thesis directed by Professor Jack D. Ives This is a historical, comparative study of the development, implementation, and results of policies for managing the forests of the Colorado Rockies and the Swiss Alps, with emphasis on two study areas in each region. The Pikes Peak (Colorado) and Davos (Switzerland) areas have been adjacent to regional urban centers since the late 19th century. The Summit (Colorado) and Aletsch (Switzerland) areas have experienced a rapid change from a resource-based to a tourism-based economy since the 1950s. The study's theoretical basis is that of common-property resources. Three primary outputs of the forests are considered: wood, recreation, and protection. The latter includes both the protection of watersheds and the protection of infrastructure and settlements from natural hazards. Forest management policies date back to the 13th century in Switzerland and the late 19th century in Colorado, but were generally unsuccessful in achieving their objectives. In the late 19th century, the early foresters in each region succeeded in placing the protection of mountain forests on regional, and then national, political agendas. In consequence, by the beginning of the 20th century, federal policies were in place to ensure the continued provision of the primary functions of the forests recognized at that time: protection and timber supply. During the 20th century, these policies have been expanded, with increasing emphasis on the provision of public goods. However, most policies have been reactive, not proactive. -

Fahrplan 2020 Leuk-Leukerbad Und Umgebung Gültig/Valable Vom/Du 15
LLBREISEN.CH Fahrplan 2020 Leuk-Leukerbad und Umgebung Gültig/Valable vom/du 15. Dezember 2019 bis 12. Dezember 2020 LLB AG, 3952 Susten Telefon +41 27 474 98 00, [email protected], www.llbreisen.ch Zeichenerklärung zu Fahrplan Verkehrstage / Liniennetzplan A Montag-Freitag ohne allg. Feiertage* / Lundi-vendredi, sauf fêtes générales B Täglich ohne Samstage / Chaque jour, sauf samedis C Samstage, Sonn- und allg. Feiertage / Samedis, dimanches et fêtes générales 1 Montag / Lundi 2 Dienstag / Mardi 3 Mittwoch / Mercredi 4 Donnerstag / Jeudi 5 Freitag / Vendredi 6 Samstag / Samedi 7 Sonntag / Dimanche Torrent der sonnigste Erlebnisberg, mitten im Wallis Haltepunkt / Arrêt Tief im Berginnern der Torrent reift das gesunde Thermalwasser von Leukerbad heran. So geheimnisvoll der Berg im inneren ist, so vielfältig ist sein Angebot an Wandern, Biken Abfahrtszeit / heure de départ und Geniessen: unsere 3 Geheimtipps im Sommer: Ankunftszeit / heure d’arrivée • Auf dem Panoramawanderweg zum Wysse See, zum Ursprung des Thermalwassers Velotransport: 48 h vor der Fahrt über www.resabike.ch (Reservationsfristen unbedingt beachten) • Einfache Gipfeltour zum Torrenthorn 2‘997 m.ü.M. mit einer 360° Bergpanoramasicht Gesellschaften und Gruppen nur nach vorheriger Anmeldung • Torrenttrail, ein Biketrail geprägt von interessanten, langen Abfahrten und kurzen Aufstiegen Der Winter macht Torrent zum aktiven Wintererlebnisberg und zum Treffpunkt für Jung & Alt: unsere 3 Geheimtipps für: Alle Kurse (Hochflurfahrzeuge mit Rollstuhlhublift oder Rampe). • Skifahrer: 55 Pistenkilometer, von einfach bis anspruchsvolle und der Talabfahrt «Berg & Tal» von 10 km und 1‘070 Höhen- Voranmeldung mindestens 2 Stunden vor Antritt der Fahrt 027 470 20 52 meter. * Allgemeine Feiertage sind: 1.1., 2.1., Karfreitag, Ostermontag, Auffahrt, Pfingstmontag, 1.8., 25.12., 26.12. -

A New Challenge for Spatial Planning: Light Pollution in Switzerland
A New Challenge for Spatial Planning: Light Pollution in Switzerland Dr. Liliana Schönberger Contents Abstract .............................................................................................................................. 3 1 Introduction ............................................................................................................. 4 1.1 Light pollution ............................................................................................................. 4 1.1.1 The origins of artificial light ................................................................................ 4 1.1.2 Can light be “pollution”? ...................................................................................... 4 1.1.3 Impacts of light pollution on nature and human health .................................... 6 1.1.4 The efforts to minimize light pollution ............................................................... 7 1.2 Hypotheses .................................................................................................................. 8 2 Methods ................................................................................................................... 9 2.1 Literature review ......................................................................................................... 9 2.2 Spatial analyses ........................................................................................................ 10 3 Results ....................................................................................................................11 -

Mitteilungsblatt 2. Quartal 2017
Mitteilungsblatt 2. Quartal 2017 Einwohnerkontrolle Im vergangenen Quartal haben sich nachfolgende Personen in der Gemeinde Eggerberg an- oder abgemeldet: Zuzüger: 01.04.2017 Schnydrig Daniel 17.04.2017 Schulz Ron 01.05.2017 Szwedowski Arkadiusz 01.05.2017 Tobias Mateusz Pawel 01.05.2017 Florek Mateusz Kamil 24.05.2017 Moore Patrick 19.06.2017 Fux Jenny Wegzüger: 05.04.2017 In-Albon Ewald 16.05.2017 Degastino Ruggiero 30.06.2017 Zimmermann Nadia Wir heissen die neuen Einwohner in Eggerberg herzlich willkommen und wünschen den Wegzügern alles Gute am neuen Wohnort. Todesfälle: Im Alter von 67 Jahren ist Erwin Wasmer am 24. April 2017 nach kurzem Spitalaufenthalt und längerer Leidenszeit im Kreise seiner Familie friedlich entschlafen. Am 20. Juni 2017 ist Lina Brunschwiler-Berchtold im Alter von 92 Jahren im Altersheim in Thal gestorben und wurde ihrem Wunsch entsprechend in Eggerberg beerdigt. Den Angehörigen entbieten wir unsere aufrichtige Anteilnahme. Der Herr gebe den Verstorbenen die ewige Ruhe. Mitteilungsblatt Gemeinde Eggerberg 2. Quartal 2017 Aktuelle Gemeindeinformationen ▒ Fronleichnam/Gemeindetrüch 2017 Am 15. Juni 2017 konnte das Fronleichnamsfest und der anschliessende Gemeindetrüch bei strahlendem Sommerwetter und herrlichen Temperaturen durchgeführt werden. Dieses Jahr konnte dem Jahrgang 1999 der Bürgerbrief überreicht und in den Kreis der vollberechtigten und mitverantwortlichen Staatsbürger aufgenommen werden. Die Gemeindeverwaltung gratuliert herzlich und hofft auf eine rege Beteiligung bei Wahlen und Abstimmungen. Mit ihrer Stimmabgabe bekunden sie, dass sie gewillt sind, Mitverantwortung zu tragen und anderseits am Wohlergehen unseres Dorfes, Kantons und der Schweiz interessiert sind. Die stolzen Jungbürger; v.l. Rafael Millius, Elena In-Albon, Maria Imesch und Diego Anthamatten mit ihrem Bürgerbrief umringt vom Gemeinderat. -

Gmeind Gemeinde Gampel-Bratsch
mitteilungsblatt der gemeinden gampel-bratsch | steg-hohtenn ausgabe 23 | Mai 2014 weibil markus fryand der turnpräsident os gampel-steg: der schuldirektor im gespräch gesundheit: arztpraxis lötschberg im wandel inhalt 1 Armin Bregy 1 2 Josef Pfammatter Sei es im Kleinen, sei es im Grossen Verzeichnis In dieser Nummer, es ist mittlerweile die 23. Ausgabe, kommen Leute aus Gampel- vorwort 3 Bratsch und Steg-Hohtenn zu Wort, die für ihre Sache einstehen und versuchen – sei es im Kleinen, sei es im Grossen – die Region vorwärts zu bringen oder zumindest zu gmeind beleben. protokollsplitter steg-hohtenn 4 Da ist zum Beispiel Maria Indermitte, die mit ihrem Team ein ambitiöses Theater- protokollsplitter projekt auf die Beine stellt. Das Stück «Don Camillo & seine Herde» wird im Sommer gampel-bratsch 6 aufgeführt. Ein kulinarischer und kultureller Leckerbissen für Herz, Kopf, Bauch und gemeindeführerstab 8 Zwerchfell. untergetwing 9 Guido Bregy ist der Präsident der Oberwalliser Feuerwehrverbandes und Kommandant wärchu der Stützpunkt-Feuerwehr. Er engagiert sich seit Jahren für die Feuerwehr. Wir fragen, arztpraxis lötschberg 10 was den Reiz des Feuerwehrwesens ausmacht und welche Herausforderungen auf den notfallpraxis 11 Kommandanten zukommen. läbu Unsere Titelgeschichte widmen wir dem STV Gampel. Der Turnverein gehört zu den georges jäger 12 grössten Vereinen der Region und ist kantonsweit ein Begriff. Präsident Markus st. anna 13 Fryand erzählt, wie er zum Turnen gekommen ist, wie er den Turnverein weiter- guido bregy 14 bringen will und er berichtet, wie es dazu kam, dass er als Leichtathlet die West- schweizer-Meisterschaften verpasste – und stattdessen in einem Musik-Laden landete. persönlich markus fryand 16 Gampel-Bratsch hat den Skilift, Steg-Hohtenn das Hallenbad. -

Agglomeration Brig-Visp-Naters
RAUM+ AGGLOMERATION BRIG-VISP-NATERS April 2013 Teil 2: Aktionsplan „Siedlungsentwicklung in der Agglomeration“ ProRaum Consult Raumplanung und Flächenmanagement Dr. Hany Elgendy Impressum Abschlussbericht Raum+ Agglomeration Brig-Visp-Naters Teil 2: Aktionsplan „Siedlungsentwicklung in der Agglomeration" April 2013 Bearbeitung ProRaum Consult Raumplanung & Flächenmanagement Ludwig-Wilhelm-Str. 10 D-76131 Karlsruhe +49 (173) 890 81 41 http://www.pro-raum-consult.com Dr. Hany Elgendy Dipl.-Ing (FH) Sina Bodmer Auftraggeber Regions- und Wirtschaftszentrum Oberwallis (RWO) Klingele Haus, Kehrstrasse 12 CH-3904 Naters Projektbegleitung RWO Ivo Nanzer (Projektleitung) Tamar Hosennen Roger Michlig Kanton Wallis Damian Jerjen Martin Bellwald Eduard Bonani Fachliche Anton Andenmatten (BSAP) Begleitung „Wie wollt ihr, ohne einen neuen Weg zu gehen, ihr selber bleiben?“ (Max Frisch, 1911 – 1991) 1. Einleitung Im ersten Teil dieses Berichtes wurden die Ergebnisse der Erhebung des Siedlungsflächenpotenzials in der Agglomeration Brig-Visp-Naters dargelegt und detailliert ausgewertet. Die Einwohnerkapazität wurde abgeschätzt und dem Bedarf gegenübergestellt. In diesem Teil des Berichts - Aktionsplan „Siedlungsentwicklung in der Agglomeration“ - stehen die Fragestellungen einer künftigen Siedlungsentwicklung in der Agglomeration sowie die geeigneten Lösungsansätze und Massnahmen zu deren Umsetzung im Vordergrund. Der Aktionsplan „Siedlungsentwicklung in der Agglomeration“ wird als strategisches Instrument eines agglomerationsweiten Flächenmanagements -

Das Freigericht Baltschieder-Gründen
www.burgerschaftvisp.ch/geschichtliches/historisches/DasFreigerichtBaltschieder-Gründen.pdf Das Freigericht Baltschieder-Gründen Das Gebiet des alten Zenden Visp war früher in vier Viertel eingeteilt. Zum ersten Viertel gehörten die Gemeinden Visp, Eyholz, Lalden, Baltschieder, Visperterminen und Zeneggen; zum zweiten Viertel Stalden, Staldenried, Grächen, Embd und Törbel; zum dritten Viertel die Talschaft Saas und die Gemeinde Eisten; und zum vierten Viertel schliesslich gehörte das Gebiet von Kipfen und die jetzigen Gemeinden Sankt Niklaus, Randa, Täsch und Zermatt. Alle vier Viertel waren im Zendenrat gleichberechtigt. Die Gerichtsbarkeit im Zenden unterteilte sich jedoch in sechs Gerichtsterritorien, von denen jede seine eigene Geschichte hat: Die Kastlanei Visp, früher Meiertum. Sie umfasste die drei Viertel Visp (Visp, Eyholz, Lalden, Visperterminen, Zeneggen, Törbel), Stalden (Stalden, Staldenried, Embd und Grächen) und Saas. Der Richter über dieses Gebiet trug den Titel “Grosskastlan der löblichen drei Viertel Visp“. Als alter Brauch wird 1682 bezeichnet, jedes Jahr um das Fest der hl. Jungfrau und Märtyrerin Katharina einen Kastlan zu setzten. Das Freigericht Baltschieder-Gründen, seit dem 17. Jh. im Besitz der Burgerschaft Visp. Das Meiertum Kipfen, gelegen zwischen Stalden und Sankt Niklaus. Das Meiertum Sankt Niklaus oder Chouson (Gasen), welches das Gebiet der heutigen Ge- meinde Sankt Niklaus umfasste. Das Meiertum Zermatt, früher Herrschaft mehrerer Familien. Die Kastlanei Täsch-Randa. © www.burgerschaftvisp.ch 1/7 N. Pfaffen, -

4055/NET A9 Info N. 5
Nr.5 > JUNI 2002 info zur Autobahn A9 Steg/Gampel - Visp Autobahn A9 Steg/Gampel - Visp sur l'autoroute A9 Das Oberwallis wartet mit Ungeduld auf Die Planung wurde durch Unsicherheiten Steg/Gampel - Visp die durchgehend befahrbare Autobahn verzögert, die den Militärflugplatz Raron zwischen Siders und Brig. Auf der und das Netz der neuen Eisenbahn- Kantonsstrasse T9 gibt es regelmässig alpentransversale NEAT betreffen. Später Staus, die Ortschaften unterliegen musste auch die dritte Rhonekorrektion hohen Lärmbelastungen, während die miteinbezogen werden. DEPARTEMENT FÜR Sicherheitsvorkehrungen für alle VERKEHR, BAU UND Benutzer ungenügend sind. Zudem Erst nachdem diese verschiedenen UMWELT (DVBU) bremst die aktuelle Verkehrssituation die Fragen geregelt waren, konnte die DES KANTON WALLIS wirtschaftliche Entwicklung der Region, Planung vorangetrieben werden. Das von DEPARTEMENT DES insbesondere diejenige von Tourismus, Professor Philippe Bovy erarbeitete TRANSPORTS, DE Industrie und Handel. Gutachten hat bestätigt, dass das durch L'EQUIPEMENT ET DE die Dienststellen des DVBU ausgearbeite- L'ENVIRONNEMENT (DTEE) DU CANTON Die Enge des Tals sowie der politische als te Projekt am geeignetsten ist, um den DU VALAIS auch der Volkswille, die Naturwerte des Anforderungen der Allgemeinheit bezüg- Kantons zu erhalten, haben die Wahl lich des Verkehrs, der Anschlüsse, der Dienststelle für Strassen- und Flussbau einer Linienführung nicht gerade erleich- Umwelt und der Wirtschaft zu genügen. Sektion Nationalstrassen tert. Die Hauptschwierigkeiten sind im Dieses Bulletin hat zum Ziel, die wesentli- Oberwallis Oberwallis auf der Höhe des Pfynwaldes chen Vorteile der vorgesehenen und bei Visp zu finden. In beiden Fällen Linienführung zusammenfassend darzu- Geschina 3900 Brig T 027 922 97 00 wurden Lösungen gefunden, die von der legen. -
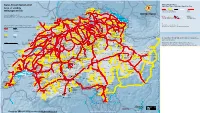
Swiss Travel System Map 2021
ai160326587010_STS-GB-Pass-S-21.pdf 1 21.10.20 09:37 Kruth Strasbourg | Paris Karlsruhe | Frankfurt | Dortmund | Hamburg | Berlin Stuttgart Ulm | München München Swiss Travel System 2021 Stockach Swiss Travel Pass Blumberg-Zollhaus Engen Swiss Travel Pass Youth | Swiss Travel Pass Flex Bargen Opfertshofen Überlingen Area of validity Seebrugg Beggingen Singen Ravensburg DEUTSCHLAND Radolfzell Schleitheim Hemmental Lines for unlimited travel (tunnel) Mulhouse Thayngen Mainau Geltungsbereich Meersburg Schaffhausen Ramsen Linien für unbegrenzte Fahrten (Tunnel) Zell (Wiesental) Wangen (Allgäu) Erzingen Oster- Neuhausen Stein a.R. Konstanz fingen Version/Stand/Etat/Stato:12.2020 (Baden) Rheinau Kreuzlingen Friedrichshafen Waldshut Due to lack of space not all lines are indicated. Subject to change. Marthalen Basel Weil a.R. Aus Platzgründen sind nicht alle Linien angegeben. Änderungen vorbehalten. Bad Zurzach Weinfelden Lines with reductions (50%, 1 25%) No reductions EuroAirport Riehen Koblenz Eglisau Frauenfeld Romanshorn Lindau Basel St.Johann Basel Möhlin Laufenburg Immenstadt Linien mit Vergünstigungen (50%, 1 25%) Keine Ermässigung Bad Bf Nieder- Stein-Säckingen Bülach Sulgen Arbon Basel Rheinfelden weningen Braunau Sonthofen Delle Pratteln Turgi Rorschach Bregenz Boncourt Ettingen Frick Brugg Zürich Bischofszell Rheineck Bonfol Liestal Baden Flughafen Winterthur Wil Rodersdorf Dornach Oberglatt Heiden St.Margrethen Aesch Gelterkinden Kloten Turbenthal St.Gallen Walzenhausen Roggenburg Wettingen Also valid for local public transport