The Evolution of Color Vision in Nocturnal Mammals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Recent Bat Survey Reveals Bukit Barisan Selatan Landscape As A
A Recent Bat Survey Reveals Bukit Barisan Selatan Landscape as a Chiropteran Diversity Hotspot in Sumatra Author(s): Joe Chun-Chia Huang, Elly Lestari Jazdzyk, Meyner Nusalawo, Ibnu Maryanto, Maharadatunkamsi, Sigit Wiantoro, and Tigga Kingston Source: Acta Chiropterologica, 16(2):413-449. Published By: Museum and Institute of Zoology, Polish Academy of Sciences DOI: http://dx.doi.org/10.3161/150811014X687369 URL: http://www.bioone.org/doi/full/10.3161/150811014X687369 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Acta Chiropterologica, 16(2): 413–449, 2014 PL ISSN 1508-1109 © Museum and Institute of Zoology PAS doi: 10.3161/150811014X687369 A recent -

Photopigments and Color Vision in the Nocturnal Monkey, Aotus GERALD H
Vision Res. Vol. 33, No. 13, pp. 1773-1783, 1993 0042-6989/93 $6.00 + 0.00 Printed in Great Britain. All rights reserved Copyright 0 1993 Pergamon Press Ltd Photopigments and Color Vision in the Nocturnal Monkey, Aotus GERALD H. JACOBS,*? JESS F. DEEGAN II,* JAY NEITZ,$ MICHAEL A. CROGNALE,§ MAUREEN NEITZT Received 6 November 1992; in revised form 3 February 1993 The owl monkey (Aotus tridrgutus) is the only nocturnal monkey. The photopigments of Aotus and the relationship between these photopigments and visual discrimination were examined through (1) an analysis of the tlicker photometric electroretinogram (ERG), (2) psychophysical tests of visual sensitivity and color vision, and (3) a search for the presence of the photopigment gene necessary for the production of a short-wavelength sensitive (SWS) photopigment. Roth electrophysiological and behavioral measurements indicate that in addition to a rod photopigment the retina of this primate contains only one other photopigment type-a cone pigment having a spectral peak cu 543 nm. Earlier results that suggested these monkeys can make crude color discriminations are interpreted as probably resulting from the joint exploitation of signals from rods and cones. Although Aotus has no functional SWS photopigment, hybridization analysis shows that A&us has a pigment gene that is highly homologous to the human SWS photopigment gene. Aotus trivirgatus Cone photopigments Monkey color vision Monochromacy Photopigment genes Evolution of color vision INTRODUCTION interest to anyone interested in visual adaptations for two somewhat contradictory reasons. On the one hand, The owl monkey (A&us) is unique among present study of A&us might provide the possibility of docu- day monkeys in several regards. -
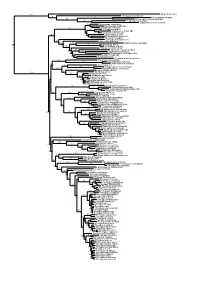
Figs1 ML Tree.Pdf
100 Megaderma lyra Rhinopoma hardwickei 71 100 Rhinolophus creaghi 100 Rhinolophus ferrumequinum 100 Hipposideros armiger Hipposideros commersoni 99 Megaerops ecaudatus 85 Megaerops niphanae 100 Megaerops kusnotoi 100 Cynopterus sphinx 98 Cynopterus horsfieldii 69 Cynopterus brachyotis 94 50 Ptenochirus minor 86 Ptenochirus wetmorei Ptenochirus jagori Dyacopterus spadiceus 99 Sphaerias blanfordi 99 97 Balionycteris maculata 100 Aethalops alecto 99 Aethalops aequalis Thoopterus nigrescens 97 Alionycteris paucidentata 33 99 Haplonycteris fischeri 29 Otopteropus cartilagonodus Latidens salimalii 43 88 Penthetor lucasi Chironax melanocephalus 90 Syconycteris australis 100 Macroglossus minimus 34 Macroglossus sobrinus 92 Boneia bidens 100 Harpyionycteris whiteheadi 69 Harpyionycteris celebensis Aproteles bulmerae 51 Dobsonia minor 100 100 80 Dobsonia inermis Dobsonia praedatrix 99 96 14 Dobsonia viridis Dobsonia peronii 47 Dobsonia pannietensis 56 Dobsonia moluccensis 29 Dobsonia anderseni 100 Scotonycteris zenkeri 100 Casinycteris ophiodon 87 Casinycteris campomaanensis Casinycteris argynnis 99 100 Eonycteris spelaea 100 Eonycteris major Eonycteris robusta 100 100 Rousettus amplexicaudatus 94 Rousettus spinalatus 99 Rousettus leschenaultii 100 Rousettus aegyptiacus 77 Rousettus madagascariensis 87 Rousettus obliviosus Stenonycteris lanosus 100 Megaloglossus woermanni 100 91 Megaloglossus azagnyi 22 Myonycteris angolensis 100 87 Myonycteris torquata 61 Myonycteris brachycephala 33 41 Myonycteris leptodon Myonycteris relicta 68 Plerotes anchietae -

Priority-Setting for Philippine Bats Using Practical Approach to Guide Effective Species Conservation and Policy-Making in the Anthropocene
Published by Associazione Teriologica Italiana Volume 30 (1): 74–83, 2019 Hystrix, the Italian Journal of Mammalogy Available online at: http://www.italian-journal-of-mammalogy.it doi:10.4404/hystrix–00172-2019 Research Article Priority-setting for Philippine bats using practical approach to guide effective species conservation and policy-making in the Anthropocene Krizler Cejuela Tanalgo1,2,3,4,∗, Alice Catherine Hughes1,3 1Landscape Ecology Group, Centre for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, Mengla, Yunnan Province 666303, People’s Republic of China 2International College, University of Chinese Academy of Sciences, Beijing 100049, People’s Republic of China 3Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Menglun, Mengla, Yunnan Province 666303, People’s Republic of China 4Department of Biological Sciences, College of Arts and Sciences, University of Southern Mindanao, Kabacan 9407, North Cotabato, the Republic of the Philippines Keywords: Abstract asian tropics endemism National level approaches to the development and implementation of effective conservation policy forest loss and practice are often challenged by limited capacity and resources. Developing relevant and hunting achievable priorities at the national level is a crucial step for effective conservation. The Philip- islands pine archipelago includes over 7000 islands and is one of only two countries considered both a oil palm global biodiversity hotspot and a megadiversity country. Yet, few studies have conducted over- arching synthesis for threats and conservation priorities of any species group. As bats make up a Article history: significant proportion of mammalian diversity in the Philippines and fulfil vital roles to maintain Received: 01/19/2019 ecosystem health and services we focus on assessing the threats and priorities to their conserva- Accepted: 24/06/2019 tion across the Philippines. -

Resource Competition Shapes Biological Rhythms and Promotes Temporal Niche
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.22.055160; this version posted April 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 2 3 Resource competition shapes biological rhythms and promotes temporal niche 4 differentiation in a community simulation 5 6 Resource competition, biological rhythms, and temporal niches 7 8 Vance Difan Gao1,2*, Sara Morley-Fletcher1,4, Stefania Maccari1,3,4, Martha Hotz Vitaterna2, Fred W. Turek2 9 10 1UMR 8576 Unité de Glycobiologie Structurale et Fonctionnelle, Campus Cité Scientifique, CNRS, University of 11 Lille, Lille, France 12 2 Center for Sleep and Circadian Biology, Northwestern University, Evanston, IL, United States of America 13 3Department of Medico-Surgical Sciences and Biotechnologies, University Sapienza of Rome, Rome, Italy 14 4International Associated Laboratory (LIA) “Perinatal Stress and Neurodegenerative Diseases”: University of Lille, 15 Lille, France; CNRS-UMR 8576, Lille, France; Sapienza University of Rome, Rome, Italy; IRCCS Neuromed, Pozzilli, 16 Italy 17 18 19 * Corresponding author 20 E-mail: [email protected] 21 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.04.22.055160; this version posted April 22, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. -

Bat Count 2003
BAT COUNT 2003 Working to promote the long term, sustainable conservation of globally threatened flying foxes in the Philippines, by developing baseline population information, increasing public awareness, and training students and protected area managers in field monitoring techniques. 1 A Terminal Report Submitted by Tammy Mildenstein1, Apolinario B. Cariño2, and Samuel Stier1 1Fish and Wildlife Biology, University of Montana, USA 2Silliman University and Mt. Talinis – Twin Lakes Federation of People’s Organizations, Diputado Extension, Sibulan, Negros Oriental, Philippines Photo by: Juan Pablo Moreiras 2 EXECUTIVE SUMMARY Large flying foxes in insular Southeast Asia are the most threatened of the Old World fruit bats due to deforestation, unregulated hunting, and little conservation commitment from local governments. Despite the fact they are globally endangered and play essential ecological roles in forest regeneration as seed dispersers and pollinators, there have been only a few studies on these bats that provide information useful to their conservation management. Our project aims to promote the conservation of large flying foxes in the Philippines by providing protected area managers with the training and the baseline information necessary to design and implement a long-term management plan for flying foxes. We focused our efforts on the globally endangered Philippine endemics, Acerodon jubatus and Acerodon leucotis, and the bats that commonly roost with them, Pteropus hypomelanus, P. vampyrus lanensis, and P. pumilus which are thought to be declining in the Philippines. Local participation is an integral part of our project. We conducted the first national training workshop on flying fox population counts and conservation at the Subic Bay area. -

Bats As Bushmeat: a Global Review S Imon M Ickleburgh,Kerry W Aylen and P Aul R Acey
Review Bats as bushmeat: a global review S imon M ickleburgh,Kerry W aylen and P aul R acey Abstract A questionnaire survey and literature review on bats. There is some evidence that hunting and trade is revealed the extent of hunting of bats for bushmeat in having a significant impact on bat populations in the Pacific the Old World tropics. High levels of offtake were reported islands and South-East Asia (Mickleburgh et al., 2002) and throughout Asia, the Pacific islands and some Western also in Madagascar (Jenkins & Racey, 2008) but there is no Indian Ocean islands, where fruit bats of the genus overall view of its potential global impact on bats. Further- Pteropus are eaten extensively. Most hunting in Africa was more, recent reviews of emergent viral diseases in bats have reported in western states and the largest fruit bat Eidolon raised concerns that eating bats as bushmeat may transmit helvum was preferred. Insectivorous bats are also eaten, such diseases (Messenger et al., 2003). particularly Tadarida in Asia. Hunting is both for local The low reproductive rate of bats makes them especially consumption and commercial, sometimes involving cross- vulnerable to harvesting for bushmeat. In several life-history border transactions. The high levels of hunting reported characteristics bats are similar to primates that are severely and the low reproductive rate of bats indicate there are impacted by the bushmeat trade (Bowen-Jones & Pendry, likely to be severe negative effects on bat populations, and 1999). Bats are long-lived and often roost communally, declines of several species are documented. Although there which increases their visibility and susceptibility to hunters. -

Index of Handbook of the Mammals of the World. Vol. 9. Bats
Index of Handbook of the Mammals of the World. Vol. 9. Bats A agnella, Kerivoula 901 Anchieta’s Bat 814 aquilus, Glischropus 763 Aba Leaf-nosed Bat 247 aladdin, Pipistrellus pipistrellus 771 Anchieta’s Broad-faced Fruit Bat 94 aquilus, Platyrrhinus 567 Aba Roundleaf Bat 247 alascensis, Myotis lucifugus 927 Anchieta’s Pipistrelle 814 Arabian Barbastelle 861 abae, Hipposideros 247 alaschanicus, Hypsugo 810 anchietae, Plerotes 94 Arabian Horseshoe Bat 296 abae, Rhinolophus fumigatus 290 Alashanian Pipistrelle 810 ancricola, Myotis 957 Arabian Mouse-tailed Bat 164, 170, 176 abbotti, Myotis hasseltii 970 alba, Ectophylla 466, 480, 569 Andaman Horseshoe Bat 314 Arabian Pipistrelle 810 abditum, Megaderma spasma 191 albatus, Myopterus daubentonii 663 Andaman Intermediate Horseshoe Arabian Trident Bat 229 Abo Bat 725, 832 Alberico’s Broad-nosed Bat 565 Bat 321 Arabian Trident Leaf-nosed Bat 229 Abo Butterfly Bat 725, 832 albericoi, Platyrrhinus 565 andamanensis, Rhinolophus 321 arabica, Asellia 229 abramus, Pipistrellus 777 albescens, Myotis 940 Andean Fruit Bat 547 arabicus, Hypsugo 810 abrasus, Cynomops 604, 640 albicollis, Megaerops 64 Andersen’s Bare-backed Fruit Bat 109 arabicus, Rousettus aegyptiacus 87 Abruzzi’s Wrinkle-lipped Bat 645 albipinnis, Taphozous longimanus 353 Andersen’s Flying Fox 158 arabium, Rhinopoma cystops 176 Abyssinian Horseshoe Bat 290 albiventer, Nyctimene 36, 118 Andersen’s Fruit-eating Bat 578 Arafura Large-footed Bat 969 Acerodon albiventris, Noctilio 405, 411 Andersen’s Leaf-nosed Bat 254 Arata Yellow-shouldered Bat 543 Sulawesi 134 albofuscus, Scotoecus 762 Andersen’s Little Fruit-eating Bat 578 Arata-Thomas Yellow-shouldered Talaud 134 alboguttata, Glauconycteris 833 Andersen’s Naked-backed Fruit Bat 109 Bat 543 Acerodon 134 albus, Diclidurus 339, 367 Andersen’s Roundleaf Bat 254 aratathomasi, Sturnira 543 Acerodon mackloti (see A. -

Misaligned Feeding Impairs Memories Dawn H Loh1,5*, Shekib a Jami2,5
1 Misaligned feeding impairs memories 2 Dawn H Loh1,5*, Shekib A Jami2,5, Richard E Flores1, Danny Truong1, Cristina A Ghiani1,3, 3 Thomas J O’Dell4,5, Christopher S Colwell1,5*. 4 5 1Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, 6 University of California Los Angeles, Los Angeles, CA 90095, USA. 7 2Molecular, Cellular, and Integrative Physiology Ph.D. Program, University of California Los 8 Angeles, Los Angeles, CA 90095, USA. 9 3Department of Pathology & Laboratory Medicine, David Geffen School of Medicine, 10 University of California Los Angeles, Los Angeles, CA 90095, USA. 11 4Department of Physiology, David Geffen School of Medicine, University of California Los 12 Angeles, Los Angeles, CA 90095, USA. 13 5UCLA Integrative Center for Learning and Memory, University of California Los Angeles, Los 14 Angeles, CA 90095, USA. 15 16 *Correspondence to: [email protected], [email protected]. 1 17 Abstract 18 Robust sleep/wake rhythms are important for health and cognitive function. Unfortunately, many 19 people are living in an environment where their circadian system is challenged by inappropriate 20 meal- or work-times. Here we scheduled food access to the sleep time and examined the impact 21 on learning and memory in mice. Under these conditions, we demonstrate that the molecular 22 clock in the master pacemaker, the suprachiasmatic nucleus (SCN), is unaltered while the 23 molecular clock in the hippocampus is synchronized by the timing of food availability. This 24 chronic circadian misalignment causes reduced hippocampal long term potentiation and total 25 CREB expression. Importantly this mis-timed feeding resulted in dramatic deficits in 26 hippocampal-dependent learning and memory. -

Investigating the Role of Bats in Emerging Zoonoses
12 ISSN 1810-1119 FAO ANIMAL PRODUCTION AND HEALTH manual INVESTIGATING THE ROLE OF BATS IN EMERGING ZOONOSES Balancing ecology, conservation and public health interest Cover photographs: Left: © Jon Epstein. EcoHealth Alliance Center: © Jon Epstein. EcoHealth Alliance Right: © Samuel Castro. Bureau of Animal Industry Philippines 12 FAO ANIMAL PRODUCTION AND HEALTH manual INVESTIGATING THE ROLE OF BATS IN EMERGING ZOONOSES Balancing ecology, conservation and public health interest Edited by Scott H. Newman, Hume Field, Jon Epstein and Carol de Jong FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2011 Recommended Citation Food and Agriculture Organisation of the United Nations. 2011. Investigating the role of bats in emerging zoonoses: Balancing ecology, conservation and public health interests. Edited by S.H. Newman, H.E. Field, C.E. de Jong and J.H. Epstein. FAO Animal Production and Health Manual No. 12. Rome. The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views of FAO. -

Taxonomy and Natural History of the Southeast Asian Fruit-Bat Genus Dyacopterus
Journal of Mammalogy, 88(2):302–318, 2007 TAXONOMY AND NATURAL HISTORY OF THE SOUTHEAST ASIAN FRUIT-BAT GENUS DYACOPTERUS KRISTOFER M. HELGEN,* DIETER KOCK,RAI KRISTIE SALVE C. GOMEZ,NINA R. INGLE, AND MARTUA H. SINAGA Division of Mammals, National Museum of Natural History (NHB 390, MRC 108), Smithsonian Institution, P.O. Box 37012, Washington, D.C. 20013-7012, USA (KMH) Forschungsinstitut Senckenberg, Senckenberganlage 25, Frankfurt, D-60325, Germany (DK) Philippine Eagle Foundation, VAL Learning Village, Ruby Street, Marfori Heights, Davao City, 8000, Philippines (RKSCG) Department of Natural Resources, Cornell University, Ithaca, NY 14853, USA (NRI) Division of Mammals, Field Museum of Natural History, Chicago, IL 60605, USA (NRI) Museum Zoologicum Bogoriense, Jl. Raya Cibinong Km 46, Cibinong 16911, Indonesia (MHS) The pteropodid genus Dyacopterus Andersen, 1912, comprises several medium-sized fruit-bat species endemic to forested areas of Sundaland and the Philippines. Specimens of Dyacopterus are sparsely represented in collections of world museums, which has hindered resolution of species limits within the genus. Based on our studies of most available museum material, we review the infrageneric taxonomy of Dyacopterus using craniometric and other comparisons. In the past, 2 species have been described—D. spadiceus (Thomas, 1890), described from Borneo and later recorded from the Malay Peninsula, and D. brooksi Thomas, 1920, described from Sumatra. These 2 nominal taxa are often recognized as species or conspecific subspecies representing these respective populations. Our examinations instead suggest that both previously described species of Dyacopterus co-occur on the Sunda Shelf—the smaller-skulled D. spadiceus in peninsular Malaysia, Sumatra, and Borneo, and the larger-skulled D. -

S00265-020-02831-2
Fear of the dark? A mesopredator mitigates large carnivore risk through ANGOR UNIVERSITY nocturnality, but humans moderate the interaction. Haswell, Peter; Kusak, Josip; Jones, Katherine; Hayward, Matt Behavioral Ecology and Sociobiology PRIFYSGOL BANGOR / B Published: 04/05/2020 Publisher's PDF, also known as Version of record Cyswllt i'r cyhoeddiad / Link to publication Dyfyniad o'r fersiwn a gyhoeddwyd / Citation for published version (APA): Haswell, P., Kusak, J., Jones, K., & Hayward, M. (2020). Fear of the dark? A mesopredator mitigates large carnivore risk through nocturnality, but humans moderate the interaction. Behavioral Ecology and Sociobiology, 74(62). http://10.1007/s00265-020-02831-2 Hawliau Cyffredinol / General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. 27. Sep. 2021 Behavioral Ecology and Sociobiology (2020) 74: 62 https://doi.org/10.1007/s00265-020-02831-2 FEATURED STUDENT RESEARCH PAPER Fear of the dark? A mesopredator mitigates large carnivore risk through nocturnality, but humans moderate the interaction Peter M.