Two New Aromatic Polyketides from a Sponge- Derived Fusarium by Agus Trianto
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

M.Sc. CHEMISTRY
M.Sc. CHEMISTRY ANALYTICAL CHEMISTRY SPECIALISATION SYLLABUS OF III & IV SEMESTERS REVISED AS PER NEW (CB) SYLLABUS FOR STUDENTS ADMITTED FROM THE YEAR 2016 ONWARDS M.Sc. CHEMISTRY (ANALYTICAL CHEMISTRY SPECIALISATION) Syllabus for III and IV Semesters (for the batches admitted in academic year 2016 & later under CBCS pattern) [Under Restructured CBCS Scheme] Grand total marks and credits (all 4 semesters) 2400 marks – 96 credits (Approved in the P.G. BOS meeting held on 01-07-2017) Semester - III (ANALYTICAL CHEMISTRY) [Under CBCS Scheme] (for the batches admitted in academic year 2016 & later under CBCS pattern) Hrs/week Internal assessment Semester exam Total Credits CH(AC)301T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)302T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)303T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)304T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)351P (LAB-I) 9 100 marks 4 CH(AC)352P (LAB-II) 9 100 marks 4 Total 600 marks 24 Semester - IV (ANALYTICAL CHEMISTRY) Hrs/week Internal assessment Semester exam Total Credits CH(AC)401T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)402T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)403T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)404T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)451P (LAB-I) 9 100 marks 4 CH(AC)452P (LAB-II) 9 100 marks 4 Total 600 marks 24 Grand total marks and credits (all 4 semesters) 2400 marks - 96 credits M.Sc.ANALYTICAL CHEMISTRY Semester III Paper I :CH (AC) 301T: CORE : Sampling, Data handling, Classical and Atomic spectral methods -
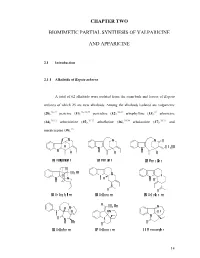
Chapter Two Biomimetic Partial Synthesis Of
CHAPTER TWO BIOMIMETIC PARTIAL SYNTHESIS OF VALPARICINE AND APPARICINE 2.1 Introduction 2.1.1 Alkaloids of Kopsia arborea A total of 62 alkaloids were isolated from the stem-bark and leaves of Kopsia arborea of which 25 are new alkaloids. Among the alkaloids isolated are valparicine (28),26,27 pericine (31),26,28,29 pericidine (32),30,31 arbophylline (33),32 arboricine (34),30,33 arboricinine (35),30,33 arboflorine (36),30,34 arboloscine (37),30,31 and mersicarpine (38).35 14 The compounds of interest to this research are valparicine (28) and pericine (31). Valparicine (28) was isolated from the stem-bark extract of K. arborea in trace amount. It represents the first member of the pericine-type alkaloids, characterized by a 16-22 exocyclic double bond, in which bond-formation has occurred between C-3 and C-7.26 Preliminary tests indicated that valparicine (28) showed strong cytotoxic effects −1 against human KB cells (IC50 < 5 μgmL ). Valparicine (28) was obtained as a colorless oil, with [α]D −40 (c 0.22, CHCl3). The UV spectrum (228 and 297 nm) indicated the presence of an unsubstituted indolenine chromophore. The EIMS of 28 showed a molecular ion at m/z 276, which 13 analyzed for C19H20N2, differing from pericine (31) by loss of two hydrogens. The C NMR spectrum gave a total of 19 carbon resonances (one methyl, five methylenes, seven methines, and six quaternary carbons) in agreement with the molecular formula. In addition to the six carbon resonances readily attributable to the aromatic moiety, and the imine resonance at δC 186.4, two other downfield quaternary resonances were observed at δC 139.2 and 144.6. -

Studies on the Synthesis and Biosynthesis Of
STUDIES ON THE SYNTHESIS AND BIOSYNTHESIS OF INDOLE ALKALOIDS BY GEORGE BOHN FULLER B.A. (cum laude) , Macalester College, 1969 M.Sc, The University of California, Berkeley, 19 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in the Department of CHEMISTRY We accept this thesis as conforming to the required standard /-) THE UNIVERSITY OF BRITISH COLUMBIA July, 1974 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the Head of my Department or by his representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Depa rtment The University of British Columbia Vancouver 8, Canada ABSTRACT Part A of this thesis provides a resume1 of the synthesis of various radioactively labelled forms of secodine C76) and provides an evaluation of these compounds, as well as some radioactively labelled forms of tryptophan C25), as precursors in the Biosynthesis of apparicine (81), uleine C83), guatam- buine (90) , and olivacine (88) in Aspidosperma australe. Only apparicine (81) could be shown to incorporate these precursors to a significant extent. Degradation of apparicine (81) from Aspidosperma pyricollum provided evidence for the intact incorporation of the secodine system. Part B discusses the synthesis of 16-epi-stemmadenine (161), which provides an entry into the stemmadenine system with, radioactive labels at key positions in the molecule. -

Total Synthesis of the Bridged Indole Alkaloid Apparicine
pubs.acs.org/joc Total Synthesis of the Bridged Indole Alkaloid Apparicine M.-Lluı¨ sa Bennasar,* Ester Zulaica, Daniel Sole, Tomas Roca, Davinia Garcı´ a-Dı´ az, and Sandra Alonso Laboratory of Organic Chemistry, Faculty of Pharmacy, and Institut de Biomedicina (IBUB), University of Barcelona, Barcelona 08028, Spain [email protected] Received September 15, 2009 An indole-templated ring-closing metathesis or a 2-indolylacyl radical cyclization constitute the central steps of two alternative approaches developed to assemble the tricyclic ABC substructure of the indole alkaloid apparicine. From this key intermediate, an intramolecular vinyl halide Heck reaction accomplished the closure of the strained 1-azabicyclo[4.2.2]decane framework of the alkaloid with concomitant incorporation of the exocyclic alkylidene substituents. Introduction Apparicine (Figure 1) is a fairly widespread monoterpenoid indole alkaloid, first isolated from Aspidosperma dasycarpon more than 40 years ago.1,2 Its structural elucidation,2 carried out by chemical degradation and early spectroscopic techni- ques, revealed a particular skeleton with a bridged 1-azabi- cyclo[4.2.2]decane framework fused to the indole ring and two exocyclic alkylidene (16-methylene and 20E-ethylidene) sub- Publication Date (Web): October 14, 2009 | doi: 10.1021/jo901986v stituents.3 Thesamearrangementwasalsofoundinvallesa- mine4 and later in a small number of alkaloids, including 16(S)- 5 6 Downloaded by UNIV OF BARCELONA on October 20, 2009 | http://pubs.acs.org hydroxy-16,22-dihydroapparicine or ervaticine, which differ from apparicine in the substitution at C-16.7 The apparicine alkaloids are biogenetically defined by the presence of only one carbon (C-6) connecting the indole 3- position and the aliphatic nitrogen, which is the result of the C-5 (1) Gilbert, B.; Duarte, A. -

Partial Purification and Properties of S-Adenosylmethionine. (R), (S
Planta Journal of Medicinal Plant Research medica Organ der Editor-in-Chief A. Baerheim-Svendsen, E. Hecker, Heidelberg J. M. Rowson, Mablethorpe Gesellschaft für E. Reinhard, Tübingen Leiden R. Hegnauer, Leiden M. v. Schantz, Helsinki Arzneipflanzen• Pharmazeutisches Institut H. Böhm, Halle W. Herz, Tallahassee K. F. Sewing, Hannover forschung Auf der Morgenstelle 8 F. Bohlmann, Berlin K. Hostettmann, Lausanne E. J. Shellard, London D-7400 Tübingen A. Cave, Chatenay-Malabry H. Inouye, Kyoto S. Shibata, Tokyo P. Delaveau, Paris M. A. Iyengar, Manipal Ch. Tamm, Basel Editorial Board Ding Guang-sheng, F. Kaiser, Mannheim W. S. Woo, Seoul H. P. T. Ammon, Tübingen Shanghai F. H. Kemper, Münster Xiao Pei-gen, Beijing W. Barz, Münster C. -J. Estler, Erlangen W. R. Kukovetz, Graz E. Reinhard, Tübingen N. Farnsworth, Chicago J. Lemli, Leuven •O. Sticher, Zürich H. Floss, Columbus Liang Xiao-tian, Beijing H. Wagner, München H. Friedrich, Münster M. Lounasmaa, Helsinki M. H. Zenk, München D. Fritz, Weihenstephan M. Luckner, Halle A. G. Gonzalez, La Laguna J. Lutomski, Poznan Advisory Board O. R. Gottlieb, Sao Paulo H. Menßen, Köln N. Anand, Lucknow E. Graf, Köln E. Noack, Düsseldorf R. Anton, Strasbourg H. Haas, Mannheim J. D. Phillipson, London Contents Volume 49,1983 ISSN 0032-0943 (f) Hippokrates Hippokrates Verlag Stuttgart II Contents 49,1983 Atta-ur-Rahman, Bashir, M.\ Isolation of New Alkaloids Cannabinoids in Phelipaea ramosa, a Parasite of Canna• from Catharanthus roseus 124 bis sativa) 250 Atta-ur-Rahman, Nisa, M., Farhi, S.: Isolation of Moenjod- aramine from Buxus papilosa 126 Galun, £., Aviv, D., Dantes, A., Freeman, A. -

A Highly Specific O-Methyltransferase for Nororientaline Synthesis Isolated from Argemone Platyceras Cell Cultures
Planta Journal of Medicinal Plant Research medica Organ der Editor-in-Chief A. Baerheim-Svendsen, E. Hecker, Heidelberg J. M. Rowson, Mablethorpe Gesellschaft für E. Reinhard, Tübingen Leiden R. Hegnauer, Leiden M. v. Schantz, Helsinki Arzneipflanzen• Pharmazeutisches Institut H. Böhm, Halle W. Herz, Tallahassee K. F. Sewing, Hannover forschung Auf der Morgenstelle 8 F. Bohlmann, Berlin K. Hostettmann, Lausanne E. J. Shellard, London D-7400 Tübingen A. Cave, Chatenay-Malabry H. Inouye, Kyoto S. Shibata, Tokyo P. Delaveau, Paris M. A. Iyengar, Manipal Ch. Tamm, Basel Editorial Board Ding Guang-sheng, F. Kaiser, Mannheim W. S. Woo, Seoul H. P. T. Ammon, Tübingen Shanghai F. H. Kemper, Münster Xiao Pei-gen, Beijing W. Barz, Münster C. -J. Estler, Erlangen W. R. Kukovetz, Graz E. Reinhard, Tübingen N. Farnsworth, Chicago J. Lemli, Leuven •O. Sticher, Zürich H. Floss, Columbus Liang Xiao-tian, Beijing H. Wagner, München H. Friedrich, Münster M. Lounasmaa, Helsinki M. H. Zenk, München D. Fritz, Weihenstephan M. Luckner, Halle A. G. Gonzalez, La Laguna J. Lutomski, Poznan Advisory Board O. R. Gottlieb, Sao Paulo H. Menßen, Köln N. Anand, Lucknow E. Graf, Köln E. Noack, Düsseldorf R. Anton, Strasbourg H. Haas, Mannheim J. D. Phillipson, London Contents Volume 49,1983 ISSN 0032-0943 (§)• Hippokrates Hippokrates Verlag Stuttgart II Contents 49,1983 Atta-ur-Rahman, Bashir, M.\ Isolation of New Alkaloids Cannabinoi'ds in Phelipaea ramosa, a Parasite of Canna- from Catharanthus roseus 124 bis sativa) 250 Atta-ur-Rahman, Nisa, M., Farhi, S.: Isolation of Moenjod- aramine from Buxus papilosa 126 Galun, £., Aviv, D., Dantes, A.y Freeman, A. -

Introduction (Pdf)
Dictionary of Natural Products on CD-ROM This introduction screen gives access to (a) a general introduction to the scope and content of DNP on CD-ROM, followed by (b) an extensive review of the different types of natural product and the way in which they are organised and categorised in DNP. You may access the section of your choice by clicking on the appropriate line below, or you may scroll through the text forwards or backwards from any point. Introduction to the DNP database page 3 Data presentation and organisation 3 Derivatives and variants 3 Chemical names and synonyms 4 CAS Registry Numbers 6 Diagrams 7 Stereochemical conventions 7 Molecular formula and molecular weight 8 Source 9 Importance/use 9 Type of Compound 9 Physical Data 9 Hazard and toxicity information 10 Bibliographic References 11 Journal abbreviations 12 Entry under review 12 Description of Natural Product Structures 13 Aliphatic natural products 15 Semiochemicals 15 Lipids 22 Polyketides 29 Carbohydrates 35 Oxygen heterocycles 44 Simple aromatic natural products 45 Benzofuranoids 48 Benzopyranoids 49 1 Flavonoids page 51 Tannins 60 Lignans 64 Polycyclic aromatic natural products 68 Terpenoids 72 Monoterpenoids 73 Sesquiterpenoids 77 Diterpenoids 101 Sesterterpenoids 118 Triterpenoids 121 Tetraterpenoids 131 Miscellaneous terpenoids 133 Meroterpenoids 133 Steroids 135 The sterols 140 Aminoacids and peptides 148 Aminoacids 148 Peptides 150 β-Lactams 151 Glycopeptides 153 Alkaloids 154 Alkaloids derived from ornithine 154 Alkaloids derived from lysine 156 Alkaloids -

Organic Chemistry Iv
FACULTY OF SCIENCE Department of Chemistry SATAVAHANA UNIVERSITY – KARIMNAGAR M.Sc. ORGANIC CHEMISTRY III & IV SEMESTER SYLLABUS (For the batch admitted during the academic year 2017-2018, Under Choice Based Credit System (CBCS)) M.Sc. (Organic Chemistry) III Semester Paper Workload Per Week Marks Duration Title Credits of the Code Theory Practical Internal External Total Exams. MCHE (OC) 301T Spectral techniques 4 -- 20 80 100 4 3 Hrs Conformational analysis, asymmetric MCHE (OC) 302T 4 -- 20 80 100 4 3 Hrs synthesis & Combinatorial chemistry MCHE (OC) 303T (E-I) Modern organic synthesis (OR) (or) 4 -- 20 80 100 4 3 Hrs MCHE (OC) 303T (E-II) Heterocyclic Compounds MCHE (OC) 304T (E-I) General Organic Chemistry (or) (OR) 4 -- 20 80 100 4 3 Hrs MCHE (OC) 304T (E-II) Natural Products Separation and identification of organic MCHE (OC) 351P -- 6 20 80 100 4 4 Hrs compounds synthesis of organic molecules and MCHE (OC) 352P -- 6 20 80 100 4 4 Hrs isolation of natural products Student Seminar 2 -- 25 -- 25 1 1 Hr TOTAL 18 12 145 480 625 25 M.Sc. (Organic Chemistry) IV Semester Paper Workload Per Week Marks Duration Title Credits of the Code Theory Practical Internal External Total Exams. MCHE (OC) 401T Drug design and Drug Discovery 4 -- 20 80 100 4 3 Hrs Reaction Mechanism, Non benzenoid MCHE (OC) 402T 4 -- 20 80 100 4 3 Hrs Aromatics and Nanomaterials Drug synthesis and mechanism of MCHE (OC) 403T (E-I) action (OR) (or) 4 -- 20 80 100 4 3 Hrs MCHE (OC) 403T (E-II) Biopharmaceutics and Pharmacodynamics MCHE (OC) 404T (E-I) Advanced Natural -

Phcogj.Com Potential Activity of Medicinal Plants As Pain Modulators
Pharmacogn J. 2021; 13(1): 248-263 A Multifaceted Journal in the field of Natural Products and Pharmacognosy Review Article www.phcogj.com Potential Activity of Medicinal Plants as Pain Modulators: A Review Carmen R. Silva-Correa1,*, Jorge L. Campos-Reyna2, Víctor E Villarreal-La Torre1, Abhel A. Calderón-Peña3, María V. González Blas1, Cinthya L. Aspajo-Villalaz3, José L. Cruzado-Razco1, William Antonio Sagástegui- Guarniz1, Luz M. Guerrero-Espino2, Julio Hilario-Vargas2 ABSTRACT This review aims to demonstrate the relevance that medicinal plants and their promising results have in prevention and treatment of pain. The neurophysiological bases of pain have been analyzed and the potential mechanisms of action have been proposed, it has also been Carmen R. Silva-Correa1,*, determined that the main experimental models used for the evaluation of the analgesic Jorge L. Campos-Reyna2, Víctor potential are: acetic acid-induced writhing test, formalin test, hot-plate test, capsaicin-induced E Villarreal-La Torre1, Abhel nociception, cinnamaldehyde-induced nociception, glutamate-induced nociception, tail–flick A. Calderón-Peña3, María V. test and tail immersion test. There are countless medicinal plants with potential analgesic González Blas1, Cinthya L. Aspajo- activity, in some of them main responsible compounds for the activity are flavonoids (vitexin, Villalaz3, José L. Cruzado-Razco1, quercetin, naringenin, astragalin, eupatilin), alkaloids (scotanamine B, bullatine A, S-(+)- William Antonio Sagástegui- dicentrine, stephalagine, lappaconitine), terpenoids (p-cymene, thymol, menthol, citronellol, Guarniz1, Luz M. Guerrero- myrcene, carvacrol, linalool) and saponins (siolmatroside I, cayaponoside D, cayaponoside B4, Espino2, Julio Hilario-Vargas2 cayaponoside A1); however, all studies have only been carried out up to pre-clinical stages. -

Erythrina Variegata (Coral Tree) Fabaceae (Legume Family)
Annex 914 Edward Balfour, Cyclopædia of India and of Eastern and Southern Asia (2d Supp.) (1862) Annex 915 “Trema species”, Firewood Crops: Shrub and Tree Species for Energy Production (1980) Annex 916 Jim Croft, “An Introduction to the Structure of Ferns and their Allies”, Australian National Botanic Gardens (1999), available at https://www.anbg.gov.au/fern/structure.html (accessed 31 May 2016) Home > Gardens | CANBR > ferns > Structure SEARCH An Introduction to the Structure of Ferns and their Allies Prepared by Jim Croft ([email protected]) Introduction Habit, Lifeform Stems, Rhizomes Leaves, fronds Sporophyte fertility Cytology . Life Cycle . Terrestrial . Growth form . Stipe . Sori . Chromosomes . Gametophyte . Epiphyte . Branching . Branching . Sporangia . Polyploidy . Sporophyte . Aquatic . Protection . Rachis . Spores . Internal . Lamina . Heterospory . Roots . Venation . Dimorphism . Polymorphism . Sporocarps . Bulbils Introduction The ferns and their allies share a lot of commom morphlogy with the other vascular plants and in many cases the same descriptive terminology is used. However, there are some fundamental and significant differences of structure unique to the pteridophytes and a specialized terminology has evolved to descdribe these. The most obvious difference between the pteridophytes and the remainder of the vascular plants is that the ferns and their allies do not produce large floral or reproductive structures that give rise to seeds which eventually develop into the next generation of plants. Pteridophytes reproduce and disperse by means of microscopic spores, the structure and development of which is every bit as intricate and amazing as the flowers of the higher plants. This outline covers the easily recognised features of the ferns and their alies and mentions many of the technical terms used to describe them. -

Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Tuberculosis
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Tuberculosis Chemical Activity Count (+)-3-HYDROXY-9-METHOXYPTEROCARPAN 1 (+)-8HYDROXYCALAMENENE 1 (+)-ALLOMATRINE 1 (+)-ALPHA-VINIFERIN 3 (+)-AROMOLINE 1 (+)-CASSYTHICINE 1 (+)-CATECHIN 10 (+)-CATECHIN-7-O-GALLATE 1 (+)-CATECHOL 1 (+)-CEPHARANTHINE 1 (+)-CYANIDANOL-3 1 (+)-EPIPINORESINOL 1 (+)-EUDESMA-4(14),7(11)-DIENE-3-ONE 1 (+)-GALBACIN 2 (+)-GALLOCATECHIN 3 (+)-HERNANDEZINE 1 (+)-ISOCORYDINE 2 (+)-PSEUDOEPHEDRINE 1 (+)-SYRINGARESINOL 1 (+)-SYRINGARESINOL-DI-O-BETA-D-GLUCOSIDE 2 (+)-T-CADINOL 1 (+)-VESTITONE 1 (-)-16,17-DIHYDROXY-16BETA-KAURAN-19-OIC 1 (-)-3-HYDROXY-9-METHOXYPTEROCARPAN 1 (-)-ACANTHOCARPAN 1 (-)-ALPHA-BISABOLOL 2 (-)-ALPHA-HYDRASTINE 1 Chemical Activity Count (-)-APIOCARPIN 1 (-)-ARGEMONINE 1 (-)-BETONICINE 1 (-)-BISPARTHENOLIDINE 1 (-)-BORNYL-CAFFEATE 2 (-)-BORNYL-FERULATE 2 (-)-BORNYL-P-COUMARATE 2 (-)-CANESCACARPIN 1 (-)-CENTROLOBINE 1 (-)-CLANDESTACARPIN 1 (-)-CRISTACARPIN 1 (-)-DEMETHYLMEDICARPIN 1 (-)-DICENTRINE 1 (-)-DOLICHIN-A 1 (-)-DOLICHIN-B 1 (-)-EPIAFZELECHIN 2 (-)-EPICATECHIN 6 (-)-EPICATECHIN-3-O-GALLATE 2 (-)-EPICATECHIN-GALLATE 1 (-)-EPIGALLOCATECHIN 4 (-)-EPIGALLOCATECHIN-3-O-GALLATE 1 (-)-EPIGALLOCATECHIN-GALLATE 9 (-)-EUDESMIN 1 (-)-GLYCEOCARPIN 1 (-)-GLYCEOFURAN 1 (-)-GLYCEOLLIN-I 1 (-)-GLYCEOLLIN-II 1 2 Chemical Activity Count (-)-GLYCEOLLIN-III 1 (-)-GLYCEOLLIN-IV 1 (-)-GLYCINOL 1 (-)-HYDROXYJASMONIC-ACID 1 (-)-ISOSATIVAN 1 (-)-JASMONIC-ACID 1 (-)-KAUR-16-EN-19-OIC-ACID 1 (-)-MEDICARPIN 1 (-)-VESTITOL 1 (-)-VESTITONE 1 -

Department of Chemistry& Pharmaceutical Sciences
DEPARTMENT OF CHEMISTRY& PHARMACEUTICAL SCIENCES MAHATMA GANDHI UNIVERSITY, NALGONDA M.Sc. Chemistry I &II Semester (CBCS) Syllabus Blow up 2017-18 Semester-I Paper-I: CH 101T (INORGANIC CHEMISTRY) IC 01: Symmetry of molecules IC 02: Bonding in Metal Complexes - I IC 03: Coordination equilibria IC 04: Ligational aspects of diatomic molecules INORGANIC CHEMISTRY Lecture No. IC-01: UNIT – 1 Symmetry of Molecules (17 Hrs.) Remarks Lecture No. 1 Concept of Symmetry in Chemistry - Symmetry Operations Lecture No. 2 Symmetry Elements : Rotational Axis of Symmetry and Types of Rotational Axes Lecture No. 3 Plane of Symmetry and Types of Planes Lecture No. 4 Improper Rotational Axis of symmetry Lecture No. 5 Inversion Center & Identity Element Lecture No. 6 More about Symmetry Elements Lecture No. 7 Molecular Point Groups: Definition and Notation of Point Groups Lecture No. 8 Classification Molecules in to C1 group Lecture No. 9 Classification of Molecules in to Cs , Ci groups Lecture No. 10 Classification of Molecules in to Cn ,Cnv groups Lecture No. 11 Classification of Molecules in to Cnh , C∞v groups Lecture No. 12 Classification of Molecules in to Dn & Dnh groups & Dnd & D∞h groups Lecture No. 13 Classification of Molecules in to Sn (n=even) T, Th & Td groups Lecture No. 14 Classification of Molecules into O & Oh groups I, Ih & Kh groups Lecture No. 15 Descent in Symmetry with Substitution & Exercises in Molecular Point Groups Lecture No. 16 Symmetry and Dipole moment Lecture No. 17 Symmetry criteria for Optical activity IC-02: UNIT-II : Bonding in metal complexes – I (16 Hrs.) Lecture No.