A Highly Specific O-Methyltransferase for Nororientaline Synthesis Isolated from Argemone Platyceras Cell Cultures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogeography of a Tertiary Relict Plant, Meconopsis Cambrica (Papaveraceae), Implies the Existence of Northern Refugia for a Temperate Herb
Article (refereed) - postprint Valtueña, Francisco J.; Preston, Chris D.; Kadereit, Joachim W. 2012 Phylogeography of a Tertiary relict plant, Meconopsis cambrica (Papaveraceae), implies the existence of northern refugia for a temperate herb. Molecular Ecology, 21 (6). 1423-1437. 10.1111/j.1365- 294X.2012.05473.x Copyright © 2012 Blackwell Publishing Ltd. This version available http://nora.nerc.ac.uk/17105/ NERC has developed NORA to enable users to access research outputs wholly or partially funded by NERC. Copyright and other rights for material on this site are retained by the rights owners. Users should read the terms and conditions of use of this material at http://nora.nerc.ac.uk/policies.html#access This document is the author’s final manuscript version of the journal article, incorporating any revisions agreed during the peer review process. Some differences between this and the publisher’s version remain. You are advised to consult the publisher’s version if you wish to cite from this article. The definitive version is available at http://onlinelibrary.wiley.com Contact CEH NORA team at [email protected] The NERC and CEH trademarks and logos (‘the Trademarks’) are registered trademarks of NERC in the UK and other countries, and may not be used without the prior written consent of the Trademark owner. 1 Phylogeography of a Tertiary relict plant, Meconopsis cambrica 2 (Papaveraceae), implies the existence of northern refugia for a 3 temperate herb 4 Francisco J. Valtueña*†, Chris D. Preston‡ and Joachim W. Kadereit† 5 *Área de Botánica, Facultad deCiencias, Universidad de Extremadura, Avda. de Elvas, s.n. -

M.Sc. CHEMISTRY
M.Sc. CHEMISTRY ANALYTICAL CHEMISTRY SPECIALISATION SYLLABUS OF III & IV SEMESTERS REVISED AS PER NEW (CB) SYLLABUS FOR STUDENTS ADMITTED FROM THE YEAR 2016 ONWARDS M.Sc. CHEMISTRY (ANALYTICAL CHEMISTRY SPECIALISATION) Syllabus for III and IV Semesters (for the batches admitted in academic year 2016 & later under CBCS pattern) [Under Restructured CBCS Scheme] Grand total marks and credits (all 4 semesters) 2400 marks – 96 credits (Approved in the P.G. BOS meeting held on 01-07-2017) Semester - III (ANALYTICAL CHEMISTRY) [Under CBCS Scheme] (for the batches admitted in academic year 2016 & later under CBCS pattern) Hrs/week Internal assessment Semester exam Total Credits CH(AC)301T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)302T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)303T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)304T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)351P (LAB-I) 9 100 marks 4 CH(AC)352P (LAB-II) 9 100 marks 4 Total 600 marks 24 Semester - IV (ANALYTICAL CHEMISTRY) Hrs/week Internal assessment Semester exam Total Credits CH(AC)401T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)402T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)403T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)404T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)451P (LAB-I) 9 100 marks 4 CH(AC)452P (LAB-II) 9 100 marks 4 Total 600 marks 24 Grand total marks and credits (all 4 semesters) 2400 marks - 96 credits M.Sc.ANALYTICAL CHEMISTRY Semester III Paper I :CH (AC) 301T: CORE : Sampling, Data handling, Classical and Atomic spectral methods -

Index Seminum Et Sporarum Quae Hortus Botanicus Universitatis Biarmiensis Pro Mutua Commutatione Offert
INDEX SEMINUM ET SPORARUM QUAE HORTUS BOTANICUS UNIVERSITATIS BIARMIENSIS PRO MUTUA COMMUTATIONE OFFERT Salix recurvigemmata A.K. Skvortsov f. variegata Schumikh., O.E. Epanch. & I.V. Belyaeva Biarmiae 2020 Federal State Autonomous Educational Institution of Higher Education «Perm State National Research University», A.G. Genkel Botanical Garden ______________________________________________________________________________________ СПИСОК СЕМЯН И СПОР, ПРЕДЛАГАЕМЫХ ДЛЯ ОБМЕНА БОТАНИЧЕСКИМ САДОМ ИМЕНИ А.Г. ГЕНКЕЛЯ ПЕРМСКОГО ГОСУДАРСТВЕННОГО НАЦИОНАЛЬНОГО ИССЛЕДОВАТЕЛЬСКОГО УНИВЕРСИТЕТА Syringa vulgaris L. ‘Красавица Москвы’ Пермь 2020 Index Seminum 2020 2 Federal State Autonomous Educational Institution of Higher Education «Perm State National Research University», A.G. Genkel Botanical Garden ______________________________________________________________________________________ Дорогие коллеги! Ботанический сад Пермского государственного национального исследовательского университета был создан в 1922 г. по инициативе и под руководством проф. А.Г. Генкеля. Здесь работали известные ученые – ботаники Д.А. Сабинин, В.И. Баранов, Е.А. Павский, внесшие своими исследованиями большой вклад в развитие биологических наук на Урале. В настоящее время Ботанический сад имени А.Г. Генкеля входит в состав регионального Совета ботанических садов Урала и Поволжья, Совет ботанических садов России, имеет статус научного учреждения и особо охраняемой природной территории. Основными научными направлениями работы являются: интродукция и акклиматизация растений, -
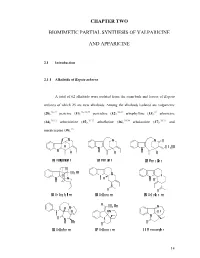
Chapter Two Biomimetic Partial Synthesis Of
CHAPTER TWO BIOMIMETIC PARTIAL SYNTHESIS OF VALPARICINE AND APPARICINE 2.1 Introduction 2.1.1 Alkaloids of Kopsia arborea A total of 62 alkaloids were isolated from the stem-bark and leaves of Kopsia arborea of which 25 are new alkaloids. Among the alkaloids isolated are valparicine (28),26,27 pericine (31),26,28,29 pericidine (32),30,31 arbophylline (33),32 arboricine (34),30,33 arboricinine (35),30,33 arboflorine (36),30,34 arboloscine (37),30,31 and mersicarpine (38).35 14 The compounds of interest to this research are valparicine (28) and pericine (31). Valparicine (28) was isolated from the stem-bark extract of K. arborea in trace amount. It represents the first member of the pericine-type alkaloids, characterized by a 16-22 exocyclic double bond, in which bond-formation has occurred between C-3 and C-7.26 Preliminary tests indicated that valparicine (28) showed strong cytotoxic effects −1 against human KB cells (IC50 < 5 μgmL ). Valparicine (28) was obtained as a colorless oil, with [α]D −40 (c 0.22, CHCl3). The UV spectrum (228 and 297 nm) indicated the presence of an unsubstituted indolenine chromophore. The EIMS of 28 showed a molecular ion at m/z 276, which 13 analyzed for C19H20N2, differing from pericine (31) by loss of two hydrogens. The C NMR spectrum gave a total of 19 carbon resonances (one methyl, five methylenes, seven methines, and six quaternary carbons) in agreement with the molecular formula. In addition to the six carbon resonances readily attributable to the aromatic moiety, and the imine resonance at δC 186.4, two other downfield quaternary resonances were observed at δC 139.2 and 144.6. -
Mohn (Papaver Somniferum
Mohn (Papaver somniferum L.) Synonyme (Namen dieser Pflanze in allen 76 Sprachen anzeigen) pharmazeutisch Semen Papaveris Albanisch Lulëkuqe Μήκων Altgriechisch Mekon ፓፓ Amharisch Papi , Arabisch , Khashkhash, Abu an-num, Abu al-num, Abu an-noom, Abu al-noom Մեկոն, Մեկոնի Կուտ Armenisch Mekon, Megon; Mekoni Kut, Megoni Good (Samen) Assamesisch Xaş-xaş Azeri Хаш-хаш Baskisch Lobelarr Bengali Градински мак, Опиев мак, Маково семе Bulgarisch Gradinski mak, Opiev mak; Makovo seme (Samen) Burmesisch Chinesisch 櫻粟殼 [yìng suhk hohk] (Kantonesisch) Ying suhk hohk Chinesisch 櫻粟殼 [yīng sù qiào], 罂粟 [yīng sù] (Mandarin) Ying su qiao, Ying su Dänisch Opiumvalmue (Pflanze); Birkes, Valmue-frø (Samen) Deutsch Schlafmohn, Gartenmohn, Ölmohn, Opiummohn, , Dhivehi Afihun, Kaskasaa Englisch Poppy, Opium poppy, Garden poppy Esperanto Papavo, Papavosemo Estnisch Magun, Unimagun, Moon Farsi Khash-khash, Shagheyegh Finnisch Unikko, Oopiumiunikko Französisch Pavot des jardins, Pavot somnifère, Pavot à opium Gälisch Codalion, Paipin Galizisch Mapoula, Sementes de Mapoula, Adormideira, Durmideira ყაყაჩო, ყაყაჩოს თესლი, ხოშხოში Georgisch Khoshkhoshi, Q’aq’acho, Xoshxoshi, Qaqacho; Q’aq’achos tesli, Qaqachos tesli (Samen) Παπαρούνα, Αθιόνι Griechisch Paparouna, Afioni Gujarati Hebräisch Pereg Hindi Irisch Poipín Isländisch Valmúafræ, Birki Italienisch Papavero, Papavero sonnifero 芥子, 罌粟 けし Japanisch ケシ, ポピー Keshi, Papi , Jiddisch Mondl, Mon Kannada Көкнәр Kasachisch Köknär Kashmiri Khash-khash Katalanisch Cascall, Herba dormidora 아편, 포피, 양귀비 Koreanisch Apyeon, Apyon, -

The Significance of Opium Alkaloids in the Classification of Papaveraceae
Journal of Pharmacognosy and Phytochemistry 2017; 6(1): 430-437 E-ISSN: 2278-4136 P-ISSN: 2349-8234 JPP 2017; 6(1): 430-437 The significance of opium alkaloids in the classification Received: 25-11-2016 Accepted: 26-12-2016 of Papaveraceae in Iraq Ula M. Noor Almousawi Department of Pharmacognosy Ula M. Noor Almousawi and Abdulrida A Alwan and medicinal plants, Pharmacy College, University of Basra, Iraq. Abstract The Papaveraceae family includes over 40 genera and 760 species in the world, of that 5 genera, namely Abdulrida A Alwan Papaver L. with 15 species, Glaucium Mill with 5 species, Hypecoum L. with 3 species, Roemeria Medic Department of Ecology, College with 2 species and Eschscholzia Cham with one cultivated species occur in Iraq. Opium alkaloids of 14 of Science, University of Basra, species of Papaveraceae in addition to two species of Fumaria L. were chemically studied using High Iraq. Performance liquid Chromatography (HPLC) analysis. Five opium alkaloids, Morphine, Codeine, Thebaine, Papaverine and Noscapine were significantly detected and variably distributed in the species. The highest concentration of opium alkaloids was Noscapine with 814.624 ppm in P. somniferum while the lowest concentration was codeine with 66.978 ppm in the same species. Our results support maintaining Fumaria in distinct family Fumariaceae. Keywords: significance, opium alkaloids, classification, Papaveraceae, Iraq 1. Introduction The Papaveraceae, known as a poppy family, are economically important. It includes over 40 [2] genera and 760 species in the world, as mentioned by Cullen (1980) 5 genera present in Iraq for Papaveraceae and 2 genera for Fumariaceae. The Papaveraceae family is characterized by its economic and medical importance from the ancient times. -

Studies on the Synthesis and Biosynthesis Of
STUDIES ON THE SYNTHESIS AND BIOSYNTHESIS OF INDOLE ALKALOIDS BY GEORGE BOHN FULLER B.A. (cum laude) , Macalester College, 1969 M.Sc, The University of California, Berkeley, 19 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in the Department of CHEMISTRY We accept this thesis as conforming to the required standard /-) THE UNIVERSITY OF BRITISH COLUMBIA July, 1974 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the Head of my Department or by his representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Depa rtment The University of British Columbia Vancouver 8, Canada ABSTRACT Part A of this thesis provides a resume1 of the synthesis of various radioactively labelled forms of secodine C76) and provides an evaluation of these compounds, as well as some radioactively labelled forms of tryptophan C25), as precursors in the Biosynthesis of apparicine (81), uleine C83), guatam- buine (90) , and olivacine (88) in Aspidosperma australe. Only apparicine (81) could be shown to incorporate these precursors to a significant extent. Degradation of apparicine (81) from Aspidosperma pyricollum provided evidence for the intact incorporation of the secodine system. Part B discusses the synthesis of 16-epi-stemmadenine (161), which provides an entry into the stemmadenine system with, radioactive labels at key positions in the molecule. -

Total Synthesis of the Bridged Indole Alkaloid Apparicine
pubs.acs.org/joc Total Synthesis of the Bridged Indole Alkaloid Apparicine M.-Lluı¨ sa Bennasar,* Ester Zulaica, Daniel Sole, Tomas Roca, Davinia Garcı´ a-Dı´ az, and Sandra Alonso Laboratory of Organic Chemistry, Faculty of Pharmacy, and Institut de Biomedicina (IBUB), University of Barcelona, Barcelona 08028, Spain [email protected] Received September 15, 2009 An indole-templated ring-closing metathesis or a 2-indolylacyl radical cyclization constitute the central steps of two alternative approaches developed to assemble the tricyclic ABC substructure of the indole alkaloid apparicine. From this key intermediate, an intramolecular vinyl halide Heck reaction accomplished the closure of the strained 1-azabicyclo[4.2.2]decane framework of the alkaloid with concomitant incorporation of the exocyclic alkylidene substituents. Introduction Apparicine (Figure 1) is a fairly widespread monoterpenoid indole alkaloid, first isolated from Aspidosperma dasycarpon more than 40 years ago.1,2 Its structural elucidation,2 carried out by chemical degradation and early spectroscopic techni- ques, revealed a particular skeleton with a bridged 1-azabi- cyclo[4.2.2]decane framework fused to the indole ring and two exocyclic alkylidene (16-methylene and 20E-ethylidene) sub- Publication Date (Web): October 14, 2009 | doi: 10.1021/jo901986v stituents.3 Thesamearrangementwasalsofoundinvallesa- mine4 and later in a small number of alkaloids, including 16(S)- 5 6 Downloaded by UNIV OF BARCELONA on October 20, 2009 | http://pubs.acs.org hydroxy-16,22-dihydroapparicine or ervaticine, which differ from apparicine in the substitution at C-16.7 The apparicine alkaloids are biogenetically defined by the presence of only one carbon (C-6) connecting the indole 3- position and the aliphatic nitrogen, which is the result of the C-5 (1) Gilbert, B.; Duarte, A. -

Partial Purification and Properties of S-Adenosylmethionine. (R), (S
Planta Journal of Medicinal Plant Research medica Organ der Editor-in-Chief A. Baerheim-Svendsen, E. Hecker, Heidelberg J. M. Rowson, Mablethorpe Gesellschaft für E. Reinhard, Tübingen Leiden R. Hegnauer, Leiden M. v. Schantz, Helsinki Arzneipflanzen• Pharmazeutisches Institut H. Böhm, Halle W. Herz, Tallahassee K. F. Sewing, Hannover forschung Auf der Morgenstelle 8 F. Bohlmann, Berlin K. Hostettmann, Lausanne E. J. Shellard, London D-7400 Tübingen A. Cave, Chatenay-Malabry H. Inouye, Kyoto S. Shibata, Tokyo P. Delaveau, Paris M. A. Iyengar, Manipal Ch. Tamm, Basel Editorial Board Ding Guang-sheng, F. Kaiser, Mannheim W. S. Woo, Seoul H. P. T. Ammon, Tübingen Shanghai F. H. Kemper, Münster Xiao Pei-gen, Beijing W. Barz, Münster C. -J. Estler, Erlangen W. R. Kukovetz, Graz E. Reinhard, Tübingen N. Farnsworth, Chicago J. Lemli, Leuven •O. Sticher, Zürich H. Floss, Columbus Liang Xiao-tian, Beijing H. Wagner, München H. Friedrich, Münster M. Lounasmaa, Helsinki M. H. Zenk, München D. Fritz, Weihenstephan M. Luckner, Halle A. G. Gonzalez, La Laguna J. Lutomski, Poznan Advisory Board O. R. Gottlieb, Sao Paulo H. Menßen, Köln N. Anand, Lucknow E. Graf, Köln E. Noack, Düsseldorf R. Anton, Strasbourg H. Haas, Mannheim J. D. Phillipson, London Contents Volume 49,1983 ISSN 0032-0943 (f) Hippokrates Hippokrates Verlag Stuttgart II Contents 49,1983 Atta-ur-Rahman, Bashir, M.\ Isolation of New Alkaloids Cannabinoids in Phelipaea ramosa, a Parasite of Canna• from Catharanthus roseus 124 bis sativa) 250 Atta-ur-Rahman, Nisa, M., Farhi, S.: Isolation of Moenjod- aramine from Buxus papilosa 126 Galun, £., Aviv, D., Dantes, A., Freeman, A. -

T.C. Republic of Turkey Hacettepe University Institute of Health Sciences Phytochemical and Pharmacological Studies on Some P
T.C. REPUBLIC OF TURKEY HACETTEPE UNIVERSITY INSTITUTE OF HEALTH SCIENCES PHYTOCHEMICAL AND PHARMACOLOGICAL STUDIES ON SOME PAPAVER SPECIES IN TURKEY Dr. MSc. Omer BAYAZEID Program of Pharmacognosy DOCTOR OF PHILOSOPHY THESIS ANKARA 2017 ii T.C. REPUBLIC OF TURKEY HACETTEPE UNIVERSITY INSTITUTE OF HEALTH SCIENCES PHYTOCHEMICAL AND PHARMACOLOGICAL STUDIES ON SOME PAPAVER SPECIES IN TURKEY Dr. MSc. Omer BAYAZEID Program of Pharmacognosy DOCTOR OF PHILOSOPHY THESIS ADVISOR OF THE THESIS Prof. Dr. Funda N. Yalçın ANKARA 2017 iii Phytochemical and Pharmacological Studies on Different Papaver Species in Turkey MSc. Pharm. Omer BAYAZEID This study has been approved and accepted as a PhD dissertation in the program of Pharmacognosy by the examining committee, whose members are listed below on 14.07.2017. Chairman of the Committee: Prof. Dr. Ayşe Uz (Faculty of Pharmacy, Hacettepe University) Advisor of the Dissertation: Prof. Dr.Funda N Yalçin (Faculty of Pharmacy, Hacettepe University) Member: Prof. Dr.Pe1in Kelicen Uğur (Faculty of Pharmacy, Hacettepe University) Member: Prof. Esra Küpeli Akkol (Faculty of Pharmacy, Gazi University Member: Prof. Dr. Omür Demirezer (Faculty of Pharmacy, Hacettepe University) This dissertation has been approved by the committee above in conformity to the regulations and by laws of Hacettepe University Graduate Programs. iv YAYIMLAMA VE FİKRİ MÜLKİYET HAKLARI BEYANI Enstitü tarafından onaylanan lisansüstü tezimin/raporumun tamamını veya herhangi bir kısmını, basılı (kağıt) ve elektronik formatta arşivleme ve aşağıda verilen koşullarla kullanıma açma iznini Hacettepe Üniversitesine verdiğimi bildiririm. Bu izinle Üniversiteye verilen kullanım hakları dışındaki tüm fikri mülkiyet haklarım bende kalacak, tezimin tamamının ya da bir bölümünün gelecekteki çalışmalarda (makale, kitap, lisans ve patent vb.) kullanım hakları bana ait olacaktır. -

Introduction (Pdf)
Dictionary of Natural Products on CD-ROM This introduction screen gives access to (a) a general introduction to the scope and content of DNP on CD-ROM, followed by (b) an extensive review of the different types of natural product and the way in which they are organised and categorised in DNP. You may access the section of your choice by clicking on the appropriate line below, or you may scroll through the text forwards or backwards from any point. Introduction to the DNP database page 3 Data presentation and organisation 3 Derivatives and variants 3 Chemical names and synonyms 4 CAS Registry Numbers 6 Diagrams 7 Stereochemical conventions 7 Molecular formula and molecular weight 8 Source 9 Importance/use 9 Type of Compound 9 Physical Data 9 Hazard and toxicity information 10 Bibliographic References 11 Journal abbreviations 12 Entry under review 12 Description of Natural Product Structures 13 Aliphatic natural products 15 Semiochemicals 15 Lipids 22 Polyketides 29 Carbohydrates 35 Oxygen heterocycles 44 Simple aromatic natural products 45 Benzofuranoids 48 Benzopyranoids 49 1 Flavonoids page 51 Tannins 60 Lignans 64 Polycyclic aromatic natural products 68 Terpenoids 72 Monoterpenoids 73 Sesquiterpenoids 77 Diterpenoids 101 Sesterterpenoids 118 Triterpenoids 121 Tetraterpenoids 131 Miscellaneous terpenoids 133 Meroterpenoids 133 Steroids 135 The sterols 140 Aminoacids and peptides 148 Aminoacids 148 Peptides 150 β-Lactams 151 Glycopeptides 153 Alkaloids 154 Alkaloids derived from ornithine 154 Alkaloids derived from lysine 156 Alkaloids -

Two New Aromatic Polyketides from a Sponge- Derived Fusarium by Agus Trianto
Two new aromatic polyketides from a sponge- derived Fusarium by Agus Trianto Submission date: 27-Jan-2020 09:53AM (UTC+0700) Submission ID: 1246808605 File name: Two_new_aromatic_polyketides_from_a.pdf (496.71K) Word count: 4972 Character count: 23548 Two new aromatic polyketides from a sponge-derived Fusarium ORIGINALITY REPORT 15% % 15% % SIMILARITY INDEX INTERNET SOURCES PUBLICATIONS STUDENT PAPERS PRIMARY SOURCES "Biosynthesis of Indocarbazostatin B, 1 % Incorporation of D-[U-13C] Glucose and L-[2- 1 13C] Tryptophan", The Journal of Antibiotics, 12/2005 Publication Peng Sun, Feng-Yuan Cai, Gianluigi Lauro, Hua 2 % Tang et al. "Immunomodulatory Biscembranoids 1 and Assignment of Their Relative and Absolute Configurations: Data Set Modulation in the Density Functional Theory/Nuclear Magnetic Resonance Approach", Journal of Natural Products, 2019 Publication You-Sheng Cai, Ariel M. Sarotti, Ting-Lan Zhou, 3 % Rong Huang et al. " Flabellipparicine, a 1 Flabelliformide-Apparicine-Type Bisindole Alkaloid from ", Journal of Natural Products, 2018 Publication Stefan Brä se, Arantxa Encinas, Julia Keck, Carl 4 % F. Nising. "Chemistry and Biology of Mycotoxins 1 and Related Fungal Metabolites", Chemical Reviews, 2009 Publication Parinda Khaengkhan, Yuki Nishikaze, Tetsuhiro 5 % Niidome, Kenji Kanaori et al. "Identification of an 1 antiamyloidogenic substance from mulberry leaves", NeuroReport, 2009 Publication G Hari Mangeswara Rao. "Studies towards the 6 % synthesis of hyperireflexolide A", Beilstein 1 Journal of Organic Chemistry, 2018 Publication María M. Zanardi, Alejandra G. Suárez, Ariel M. 7 % Sarotti. "Determination of the Relative <1 Configuration of Terminal and Spiroepoxides by Computational Methods. Advantages of the Inclusion of Unscaled Data", The Journal of Organic Chemistry, 2016 Publication Prompanya, Chadaporn, Tida Dethoup, Luís 8 % Gales, Michael Lee, José Pereira, Artur Silva, <1 Madalena Pinto, and Anake Kijjoa.