Partial Purification and Properties of S-Adenosylmethionine. (R), (S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

“Biosynthesis of Morphine in Mammals”
“Biosynthesis of Morphine in Mammals” D i s s e r t a t i o n zur Erlangung des akademischen Grades Doctor rerum naturalium (Dr. rer. nat.) vorgelegt der Naturwissenschaftlichen Fakultät I Biowissenschaften der Martin-Luther-Universität Halle-Wittenberg von Frau Nadja Grobe geb. am 21.08.1981 in Querfurt Gutachter /in 1. 2. 3. Halle (Saale), Table of Contents I INTRODUCTION ........................................................................................................1 II MATERIAL & METHODS ........................................................................................ 10 1 Animal Tissue ....................................................................................................... 10 2 Chemicals and Enzymes ....................................................................................... 10 3 Bacteria and Vectors ............................................................................................ 10 4 Instruments ........................................................................................................... 11 5 Synthesis ................................................................................................................ 12 5.1 Preparation of DOPAL from Epinephrine (according to DUNCAN 1975) ................. 12 5.2 Synthesis of (R)-Norlaudanosoline*HBr ................................................................. 12 5.3 Synthesis of [7D]-Salutaridinol and [7D]-epi-Salutaridinol ..................................... 13 6 Application Experiments ..................................................................................... -

M.Sc. CHEMISTRY
M.Sc. CHEMISTRY ANALYTICAL CHEMISTRY SPECIALISATION SYLLABUS OF III & IV SEMESTERS REVISED AS PER NEW (CB) SYLLABUS FOR STUDENTS ADMITTED FROM THE YEAR 2016 ONWARDS M.Sc. CHEMISTRY (ANALYTICAL CHEMISTRY SPECIALISATION) Syllabus for III and IV Semesters (for the batches admitted in academic year 2016 & later under CBCS pattern) [Under Restructured CBCS Scheme] Grand total marks and credits (all 4 semesters) 2400 marks – 96 credits (Approved in the P.G. BOS meeting held on 01-07-2017) Semester - III (ANALYTICAL CHEMISTRY) [Under CBCS Scheme] (for the batches admitted in academic year 2016 & later under CBCS pattern) Hrs/week Internal assessment Semester exam Total Credits CH(AC)301T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)302T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)303T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)304T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)351P (LAB-I) 9 100 marks 4 CH(AC)352P (LAB-II) 9 100 marks 4 Total 600 marks 24 Semester - IV (ANALYTICAL CHEMISTRY) Hrs/week Internal assessment Semester exam Total Credits CH(AC)401T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)402T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)403T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)404T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)451P (LAB-I) 9 100 marks 4 CH(AC)452P (LAB-II) 9 100 marks 4 Total 600 marks 24 Grand total marks and credits (all 4 semesters) 2400 marks - 96 credits M.Sc.ANALYTICAL CHEMISTRY Semester III Paper I :CH (AC) 301T: CORE : Sampling, Data handling, Classical and Atomic spectral methods -

1. Introduction
Introduction 1. Introduction 1.1. Alkaloids The term alkaloid is derived from Arabic word al-qali, the plant from which “soda” was first obtained (Kutchan, 1995). Alkaloids are a group of naturally occurring low-molecular weight nitrogenous compounds found in about 20% of plant species. The majority of alkaloids in plants are derived from the amino acids tyrosine, tryptophan and phenylalanine. They are often basic and contain nitrogen in a heterocyclic ring. The classification of alkaloids is based on their carbon-nitrogen skeletons; common alkaloid ring structures include the pyridines, pyrroles, indoles, pyrrolidines, isoquinolines and piperidines (Petterson et al., 1991; Bennett et al., 1994). In nature, plant alkaloids are mainly involved in plant defense against herbivores and pathogens. Many of these compounds have biological activity which makes them suitable for use as stimulants (nicotine, caffeine), pharmaceuticals (vinblastine), narcotics (cocaine, morphine) and poisons (tubocurarine). The discovery of morphine by the German pharmacist Friedrich W. Sertürner in 1806 began the field of plant alkaloid biochemistry. However, the structure of morphine was not determined until 1952 due to its stereochemical complexity. Major technical advances occurred in this field allowing for the elucidation of selected alkaloid biosynthetic pathways. Among these were the introduction of radiolabeled precursors in the 1950s and the establishment in the 1970s of plant cell suspension cultures as an abundant source of enzymes that could be isolated, purified and characterized. Finally, the introduction of molecular techniques has made possible the isolation of genes involved in alkaloid secondary pathways (Croteau et al., 2000; Facchini, 2001). 1.1.1. Benzylisoquinoline alkaloids Isoquinoline alkaloids represent a large and varied group of physiologically active natural products. -
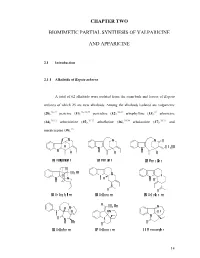
Chapter Two Biomimetic Partial Synthesis Of
CHAPTER TWO BIOMIMETIC PARTIAL SYNTHESIS OF VALPARICINE AND APPARICINE 2.1 Introduction 2.1.1 Alkaloids of Kopsia arborea A total of 62 alkaloids were isolated from the stem-bark and leaves of Kopsia arborea of which 25 are new alkaloids. Among the alkaloids isolated are valparicine (28),26,27 pericine (31),26,28,29 pericidine (32),30,31 arbophylline (33),32 arboricine (34),30,33 arboricinine (35),30,33 arboflorine (36),30,34 arboloscine (37),30,31 and mersicarpine (38).35 14 The compounds of interest to this research are valparicine (28) and pericine (31). Valparicine (28) was isolated from the stem-bark extract of K. arborea in trace amount. It represents the first member of the pericine-type alkaloids, characterized by a 16-22 exocyclic double bond, in which bond-formation has occurred between C-3 and C-7.26 Preliminary tests indicated that valparicine (28) showed strong cytotoxic effects −1 against human KB cells (IC50 < 5 μgmL ). Valparicine (28) was obtained as a colorless oil, with [α]D −40 (c 0.22, CHCl3). The UV spectrum (228 and 297 nm) indicated the presence of an unsubstituted indolenine chromophore. The EIMS of 28 showed a molecular ion at m/z 276, which 13 analyzed for C19H20N2, differing from pericine (31) by loss of two hydrogens. The C NMR spectrum gave a total of 19 carbon resonances (one methyl, five methylenes, seven methines, and six quaternary carbons) in agreement with the molecular formula. In addition to the six carbon resonances readily attributable to the aromatic moiety, and the imine resonance at δC 186.4, two other downfield quaternary resonances were observed at δC 139.2 and 144.6. -

Structure of a Berberine Bridge Enzyme-Like Enzyme with an Active Site Specific to the Plant Family Brassicaceae
Structure of a Berberine Bridge Enzyme-Like Enzyme with an Active Site Specific to the Plant Family Brassicaceae Daniel, Bastian; Wallner, Silvia; Steiner, Barbara; Oberdorfer, Gustav; Kumar, Prashant; van der Graaff, Eric; Roitsch, Thomas; Sensen, Christoph W; Gruber, Karl; Macheroux, Peter Published in: PLOS ONE DOI: 10.1371/journal.pone.0156892 Publication date: 2016 Document version Publisher's PDF, also known as Version of record Citation for published version (APA): Daniel, B., Wallner, S., Steiner, B., Oberdorfer, G., Kumar, P., van der Graaff, E., ... Macheroux, P. (2016). Structure of a Berberine Bridge Enzyme-Like Enzyme with an Active Site Specific to the Plant Family Brassicaceae. PLOS ONE, 11(6), e0156892. https://doi.org/10.1371/journal.pone.0156892 Download date: 08. Apr. 2020 RESEARCH ARTICLE Structure of a Berberine Bridge Enzyme-Like Enzyme with an Active Site Specific to the Plant Family Brassicaceae Bastian Daniel1, Silvia Wallner1, Barbara Steiner1, Gustav Oberdorfer2, Prashant Kumar2, Eric van der Graaff3, Thomas Roitsch3,4, Christoph W. Sensen5, Karl Gruber2, Peter Macheroux1* 1 Institute of Biochemistry, Graz University of Technology, Graz, Austria, 2 Institute of Molecular Biosciences, University of Graz, Graz, Austria, 3 Department of Plant and Environmental Sciences, a11111 University of Copenhagen, Copenhagen, Denmark, 4 Global Change Research Centre, Czech Globe AS CR, v.v.i., Drásov 470, Cz-664 24 Drásov, Czech Republic, 5 Institute of Molecular Biotechnology, Graz University of Technology, Graz, Austria * [email protected] OPEN ACCESS Abstract Citation: Daniel B, Wallner S, Steiner B, Oberdorfer Berberine bridge enzyme-like (BBE-like) proteins form a multigene family (pfam 08031), G, Kumar P, van der Graaff E, et al. -

Dr. Duke's Phytochemical and Ethnobotanical Databases Chemicals Found in Papaver Somniferum
Dr. Duke's Phytochemical and Ethnobotanical Databases Chemicals found in Papaver somniferum Activities Count Chemical Plant Part Low PPM High PPM StdDev Refernce Citation 0 (+)-LAUDANIDINE Fruit -- 0 (+)-RETICULINE Fruit -- 0 (+)-RETICULINE Latex Exudate -- 0 (-)-ALPHA-NARCOTINE Inflorescence -- 0 (-)-NARCOTOLINE Inflorescence -- 0 (-)-SCOULERINE Latex Exudate -- 0 (-)-SCOULERINE Plant -- 0 10-HYDROXYCODEINE Latex Exudate -- 0 10-NONACOSANOL Latex Exudate Chemical Constituents of Oriental Herbs (3 diff. books) 0 13-OXOCRYPTOPINE Plant -- 0 16-HYDROXYTHEBAINE Plant -- 0 20-HYDROXY- Fruit 36.0 -- TRICOSANYLCYCLOHEXA NE 0 4-HYDROXY-BENZOIC- Pericarp -- ACID 0 4-METHYL-NONACOSANE Fruit 3.2 -- 0 5'-O- Plant -- DEMETHYLNARCOTINE 0 5-HYDROXY-3,7- Latex Exudate -- DIMETHOXYPHENANTHRE NE 0 6- Plant -- ACTEONLYDIHYDROSANG UINARINE 0 6-METHYL-CODEINE Plant Father Nature's Farmacy: The aggregate of all these three-letter citations. 0 6-METHYL-CODEINE Fruit -- 0 ACONITASE Latex Exudate -- 32 AESCULETIN Pericarp -- 3 ALANINE Seed 11780.0 12637.0 0.5273634907250652 -- Activities Count Chemical Plant Part Low PPM High PPM StdDev Refernce Citation 0 ALKALOIDS Latex Exudate 50000.0 250000.0 ANON. 1948-1976. The Wealth of India raw materials. Publications and Information Directorate, CSIR, New Delhi. 11 volumes. 5 ALLOCRYPTOPINE Plant Father Nature's Farmacy: The aggregate of all these three-letter citations. 15 ALPHA-LINOLENIC-ACID Seed 1400.0 5564.0 -0.22115561650586155 -- 2 ALPHA-NARCOTINE Plant Jeffery B. Harborne and H. Baxter, eds. 1983. Phytochemical Dictionary. A Handbook of Bioactive Compounds from Plants. Taylor & Frost, London. 791 pp. 17 APOMORPHINE Plant Father Nature's Farmacy: The aggregate of all these three-letter citations. 0 APOREINE Fruit -- 0 ARABINOSE Fruit ANON. -

Redalyc.Identification of Isoquinoline Alkaloids from Berberis Microphylla
Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas ISSN: 0717-7917 [email protected] Universidad de Santiago de Chile Chile MANOSALVA, Loreto; MUTIS, Ana; DÍAZ, Juan; URZÚA, Alejandro; FAJARDO, Víctor; QUIROZ, Andrés Identification of isoquinoline alkaloids from Berberis microphylla by HPLC ESI-MS/MS Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, vol. 13, núm. 4, 2014, pp. 324-335 Universidad de Santiago de Chile Santiago, Chile Available in: http://www.redalyc.org/articulo.oa?id=85631435002 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative © 2014 Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 13 (4): 324 - 335 ISSN 0717 7917 www.blacpma.usach.cl Artículo Original | Original Article In memorian Professor Luis Astudillo, Universidad de Talca, Chile Identification of isoquinoline alkaloids from Berberis microphylla by HPLC ESI-MS/MS [Identificación de alcaloides isoquinolínicos en Berberis microphylla G. Forst mediante CLAE IES-MS/MS] Loreto MANOSALVA1, Ana MUTIS2, Juan DÍAZ3, Alejandro URZÚA4, Víctor FAJARDO5 & Andrés QUIROZ2 1Doctorado en Ciencias de Recursos Naturales; 2Laboratorio de Ecología Química, Departamento de Ciencias Químicas y Recursos Naturales; 3Laboratory of Mass Spectrometry, Scientific and Technological Bioresource Nucleus (Bioren), Universidad de La Frontera, Temuco, Chile 4Laboratory of Chemical Ecology, Department of Environmental Sciences, Faculty of Chemistry and Biology, Universidad de Santiago de Chile 5Chile Laboratorio de Productos Naturales, Universidad de Magallanes, Punta Arenas, Chile Contactos | Contacts: Andrés QUIROZ - E-mail address: [email protected] Abstract: Berberis microphylla (G. -

Diversity of the Mountain Flora of Central Asia with Emphasis on Alkaloid-Producing Plants
diversity Review Diversity of the Mountain Flora of Central Asia with Emphasis on Alkaloid-Producing Plants Karimjan Tayjanov 1, Nilufar Z. Mamadalieva 1,* and Michael Wink 2 1 Institute of the Chemistry of Plant Substances, Academy of Sciences, Mirzo Ulugbek str. 77, 100170 Tashkent, Uzbekistan; [email protected] 2 Institute of Pharmacy and Molecular Biotechnology, Heidelberg University, Im Neuenheimer Feld 364, 69120 Heidelberg, Germany; [email protected] * Correspondence: [email protected]; Tel.: +9-987-126-25913 Academic Editor: Ipek Kurtboke Received: 22 November 2016; Accepted: 13 February 2017; Published: 17 February 2017 Abstract: The mountains of Central Asia with 70 large and small mountain ranges represent species-rich plant biodiversity hotspots. Major mountains include Saur, Tarbagatai, Dzungarian Alatau, Tien Shan, Pamir-Alai and Kopet Dag. Because a range of altitudinal belts exists, the region is characterized by high biological diversity at ecosystem, species and population levels. In addition, the contact between Asian and Mediterranean flora in Central Asia has created unique plant communities. More than 8100 plant species have been recorded for the territory of Central Asia; about 5000–6000 of them grow in the mountains. The aim of this review is to summarize all the available data from 1930 to date on alkaloid-containing plants of the Central Asian mountains. In Saur 301 of a total of 661 species, in Tarbagatai 487 out of 1195, in Dzungarian Alatau 699 out of 1080, in Tien Shan 1177 out of 3251, in Pamir-Alai 1165 out of 3422 and in Kopet Dag 438 out of 1942 species produce alkaloids. The review also tabulates the individual alkaloids which were detected in the plants from the Central Asian mountains. -

Synthesis of Novel Compounds Based on Reticuline Scaffold for New Drugs Discovery Tam-Dan Batenburg-Nguyen University of Wollongong
University of Wollongong Research Online University of Wollongong Thesis Collection University of Wollongong Thesis Collections 2005 Synthesis of novel compounds based on reticuline scaffold for new drugs discovery Tam-Dan Batenburg-Nguyen University of Wollongong Recommended Citation Batenburg-Nguyen, Tam-Dan, Synthesis of novel compounds based on reticuline scaffold for new drugs discovery, Doctor of Philosophy thesis, Department of Chemistry, Faculty of Science, University of Wollongong, 2005. http://ro.uow.edu.au/theses/1190 Research Online is the open access institutional repository for the University of Wollongong. For further information contact Manager Repository Services: [email protected]. Synthesis of Novel Compounds Based on the Reticuline Scaffold for New Drugs Discovery. A thesis submitted in fulfilment of the requirements for the award of the degree of Doctor of Philosophy From University of Wollongong Tam-Dan (Uta) Batenburg-Nguyen B. Adv. Med Chem (Hons) Department of Chemistry University of Wollongong Wollongong, Australia December, 2005 i Declaration I, Tam-Dan (Uta) Batenburg-Nguyen hereby declare that all materials presented in this thesis, submitted in the fulfillment of the requirements for the award of Doctor of Philosophy, in the Department of Chemistry, University of Wollongong, are exclusively of my own work. These materials have not been submitted for qualifications at any other academic institution, unless otherwise referenced or acknowledged. Tam-Dan (Uta) Batenburg-Nguyen December, 2005 ii Table of Contents DECLARATION…………………………………………………………………………… i LIST OF FIGURES………………………………………………………………………….xi LIST OF SCHEMES…………………………………………………………………xiv LIST OF TABLES………………………………………………………………………… .xx LIST OF ABBREVIATIONS…………………………………………………………… xxii ABSTRACT…………………………………………………………………………… xxviii ACKNOWLEDGEMENTS……………………………………………………………… xxxii CHAPTER 1 INTRODUCTION ............................................................................... 1 1.1. HISTORY OF NATURAL PRODUCTS. .............................................................. 2 1.2. -

WO 2016/149821 Al 29 September 2016 (29.09.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/149821 Al 29 September 2016 (29.09.2016) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, C12N 9/02 (2006.01) C12N 15/81 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, C12N 1/19 (2006.01) C12N 9/04 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, C12N 15/53 (2006.01) CI2P 17/10 (2006.01) HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, C12N 15/54 (2006.01) C12P 17/12 (2006.01) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (21) International Application Number: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, PCT/CA2016/050334 SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (22) International Filing Date: TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. 23 March 2016 (23.03.2016) (84) Designated States (unless otherwise indicated, for every (25) Filing Language: English kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, (26) Publication Language: English TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (30) Priority Data: TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, 62/136,912 23 March 2015 (23.03.2015) US DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, (71) Applicant: VALORBEC SOCIETE EN COMMAN¬ SM, TR), OAPI (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, DITE [CA/CA]; 355 Peel, Carrefour INGO, Suite 503, GW, KM, ML, MR, NE, SN, TD, TG). -

Modulatory Effects of Eschscholzia Californica Alkaloids on Recombinant GABAA Receptors
Hindawi Publishing Corporation Biochemistry Research International Volume 2015, Article ID 617620, 9 pages http://dx.doi.org/10.1155/2015/617620 Research Article Modulatory Effects of Eschscholzia californica Alkaloids on Recombinant GABAA Receptors Milan Fedurco,1 Jana Gregorová,2 Kristýna Šebrlová,2 Jana Kantorová,2 Ondlej Peš,2 Roland Baur,3 Erwin Sigel,3 and Eva Táborská2 1 Michelin Recherche et Technique S.A., Route Andre-Piller´ 30, 1762 Givisiez, Switzerland 2Department of Biochemistry, Faculty of Medicine, Masaryk University, 62500 Brno, Czech Republic 3Institute of Biochemistry and Molecular Medicine, University of Bern, Buhlstrasse¨ 28, 3012 Bern, Switzerland Correspondence should be addressed to Milan Fedurco; [email protected] Received 28 July 2015; Revised 5 September 2015; Accepted 15 September 2015 Academic Editor: Emanuel Strehler Copyright © 2015 Milan Fedurco et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The California poppy (Eschscholzia californica Cham.) contains a variety of natural compounds including several alkaloids found exclusively in this plant. Because of the sedative, anxiolytic, and analgesic effects, this herb is currently sold in pharmacies in many countries. However, our understanding of these biological effects at the molecular level is still lacking. Alkaloids detected in E. californica could be hypothesized to act at GABAA receptors, which are widely expressed in the brain mainly at the inhibitory interneurons. Electrophysiological studies on a recombinant 1 2 2 GABAA receptor showed no effect of N-methyllaurotetanine at concentrations lower than 30 M. However, ()-reticuline behaved as positive allosteric modulator at the 3, 5,and6 isoforms of GABAA receptors. -

Research Article Modulatory Effects of Eschscholzia Californica Alkaloids on Recombinant GABAA Receptors
Hindawi Publishing Corporation Biochemistry Research International Volume 2015, Article ID 617620, 9 pages http://dx.doi.org/10.1155/2015/617620 Research Article Modulatory Effects of Eschscholzia californica Alkaloids on Recombinant GABAA Receptors Milan Fedurco,1 Jana Gregorová,2 Kristýna Šebrlová,2 Jana Kantorová,2 Ondlej Peš,2 Roland Baur,3 Erwin Sigel,3 and Eva Táborská2 1 Michelin Recherche et Technique S.A., Route Andre-Piller´ 30, 1762 Givisiez, Switzerland 2Department of Biochemistry, Faculty of Medicine, Masaryk University, 62500 Brno, Czech Republic 3Institute of Biochemistry and Molecular Medicine, University of Bern, Buhlstrasse¨ 28, 3012 Bern, Switzerland Correspondence should be addressed to Milan Fedurco; [email protected] Received 28 July 2015; Revised 5 September 2015; Accepted 15 September 2015 Academic Editor: Emanuel Strehler Copyright © 2015 Milan Fedurco et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The California poppy (Eschscholzia californica Cham.) contains a variety of natural compounds including several alkaloids found exclusively in this plant. Because of the sedative, anxiolytic, and analgesic effects, this herb is currently sold in pharmacies in many countries. However, our understanding of these biological effects at the molecular level is still lacking. Alkaloids detected in E. californica could be hypothesized to act at GABAA receptors, which are widely expressed in the brain mainly at the inhibitory interneurons. Electrophysiological studies on a recombinant 1 2 2 GABAA receptor showed no effect of N-methyllaurotetanine at concentrations lower than 30 M. However, ()-reticuline behaved as positive allosteric modulator at the 3, 5,and6 isoforms of GABAA receptors.