Studies on the Synthesis and Biosynthesis Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

M.Sc. CHEMISTRY
M.Sc. CHEMISTRY ANALYTICAL CHEMISTRY SPECIALISATION SYLLABUS OF III & IV SEMESTERS REVISED AS PER NEW (CB) SYLLABUS FOR STUDENTS ADMITTED FROM THE YEAR 2016 ONWARDS M.Sc. CHEMISTRY (ANALYTICAL CHEMISTRY SPECIALISATION) Syllabus for III and IV Semesters (for the batches admitted in academic year 2016 & later under CBCS pattern) [Under Restructured CBCS Scheme] Grand total marks and credits (all 4 semesters) 2400 marks – 96 credits (Approved in the P.G. BOS meeting held on 01-07-2017) Semester - III (ANALYTICAL CHEMISTRY) [Under CBCS Scheme] (for the batches admitted in academic year 2016 & later under CBCS pattern) Hrs/week Internal assessment Semester exam Total Credits CH(AC)301T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)302T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)303T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)304T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)351P (LAB-I) 9 100 marks 4 CH(AC)352P (LAB-II) 9 100 marks 4 Total 600 marks 24 Semester - IV (ANALYTICAL CHEMISTRY) Hrs/week Internal assessment Semester exam Total Credits CH(AC)401T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)402T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)403T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)404T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)451P (LAB-I) 9 100 marks 4 CH(AC)452P (LAB-II) 9 100 marks 4 Total 600 marks 24 Grand total marks and credits (all 4 semesters) 2400 marks - 96 credits M.Sc.ANALYTICAL CHEMISTRY Semester III Paper I :CH (AC) 301T: CORE : Sampling, Data handling, Classical and Atomic spectral methods -

European Journal of Biomedical and Pharmaceutical Sciences
ejbps, 2016, Volume 3, Issue 1, 62-83. Review Article SJIF Impact Factor 2.062 Kumar et al. European Journal European ofJournal Biomedical of Biomedical and Pharmaceutical ISSNSciences 2349 -8870 Volume: 3 AND Pharmaceutical sciences Issue: 1 62-83 http://www.ejbps.com Year: 2016 A REVIEW ON THE PHYTOCONSTITUENTS AND PHARMACOLOGICAL ACTIONS IN THE MEDICINAL PLANTS OF BEDABUNA FOREST, JIMMA ZONE, SOUTH WEST ETHIOPIA REPORTED EFFECT ON EXPERIMENTAL MODELS Kumar Ganesan1*, Suresh Kumar P. Nair1, Melese Sinaga1, Sharmila Banu Gani2* 1Department of Biomedical Sciences, School of Public Health and Medical Sciences, Jimma University, Jimma 378, Ethiopia 2Department of Zoology, NKR Government Arts College for Women, Namakkal-637001, Tamilnadu, India *Author for Correspondence: Dr. Kumar Ganesan Department of Biomedical Sciences, School of Public Health and Medical Sciences, Jimma University, Jimma 378, Ethiopia Article Received on 03/11/2015 Article Revised on 24/11/2015 Article Accepted on 15/12/2015 ABSTRACT Ethiopia is sixth largest biodiversity centre in the world having numerous ethinic cultures, climate and topographies. The present paper reviews on medicinal properties along with atypical Phytoconstituents and pharmacological actions of various plants in bedabuna forest, Zimma zone, Southwest Ethiopia, which has been reported effect on experimental models. This study is very authentic and helpful to find richest bioresources like identification of medicinal plants, documentation, protection and sustainable usages. This study will helpful to not only a native people of Jimma, southwest Ethiopia but also the other part of the Ethiopia to explore the indigenous medicinal plants used in the treatment of various ailments for human and livestock. In the present study totally 49 species of traditional medicinal plants belonging to 31 families were come across by regular ground visits and arbitrarily interviewed with native participants. -
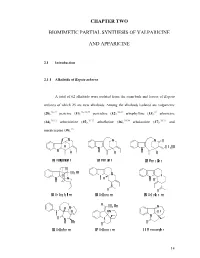
Chapter Two Biomimetic Partial Synthesis Of
CHAPTER TWO BIOMIMETIC PARTIAL SYNTHESIS OF VALPARICINE AND APPARICINE 2.1 Introduction 2.1.1 Alkaloids of Kopsia arborea A total of 62 alkaloids were isolated from the stem-bark and leaves of Kopsia arborea of which 25 are new alkaloids. Among the alkaloids isolated are valparicine (28),26,27 pericine (31),26,28,29 pericidine (32),30,31 arbophylline (33),32 arboricine (34),30,33 arboricinine (35),30,33 arboflorine (36),30,34 arboloscine (37),30,31 and mersicarpine (38).35 14 The compounds of interest to this research are valparicine (28) and pericine (31). Valparicine (28) was isolated from the stem-bark extract of K. arborea in trace amount. It represents the first member of the pericine-type alkaloids, characterized by a 16-22 exocyclic double bond, in which bond-formation has occurred between C-3 and C-7.26 Preliminary tests indicated that valparicine (28) showed strong cytotoxic effects −1 against human KB cells (IC50 < 5 μgmL ). Valparicine (28) was obtained as a colorless oil, with [α]D −40 (c 0.22, CHCl3). The UV spectrum (228 and 297 nm) indicated the presence of an unsubstituted indolenine chromophore. The EIMS of 28 showed a molecular ion at m/z 276, which 13 analyzed for C19H20N2, differing from pericine (31) by loss of two hydrogens. The C NMR spectrum gave a total of 19 carbon resonances (one methyl, five methylenes, seven methines, and six quaternary carbons) in agreement with the molecular formula. In addition to the six carbon resonances readily attributable to the aromatic moiety, and the imine resonance at δC 186.4, two other downfield quaternary resonances were observed at δC 139.2 and 144.6. -

Crystal Structure of Akuammicine, an Indole Alkaloid from Catharanthus Roseus
research communications Crystal structure of akuammicine, an indole alkaloid from Catharanthus roseus ISSN 2056-9890 Mahdi Yahyazadeh,a‡ Gerold Jerz,b Dirk Selmar,a Peter Winterhalterb and Peter G. Jonesc* aInstitut fu¨r Pflanzenbiologie, Technische Universita¨t Braunschweig, Mendelssohnstrasse 4, 38106 Braunschweig, b Received 28 September 2017 Germany, Institut fu¨r Lebensmittelchemie, Technische Universita¨t Braunschweig, Schleinitzstrasse 20, 38106 c Accepted 9 October 2017 Braunschweig, Germany, and Institut fu¨r Anorganische und Analytische Chemie, Technische Universita¨t Braunschweig, Hagenring 30, 38106 Braunschweig, Germany. *Correspondence e-mail: [email protected] Edited by D. Chopra, Indian Institute of Science The title compound, C20H22N2O2, an alkaloid isolated from the Madagascar Education and Research Bhopal, India periwinkle, crystallizes in P1 with two independent but closely similar molecules in the unit cell. The molecules are linked into pairs by two N—HÁÁÁO C ‡ On leave from Yasouj University, Yasouj, hydrogen bonds. The absolute configuration was confirmed by anomalous Kohgiluyeh Va Boyer Ahmad, Iran. dispersion effects as S at the 3 and 15 positions, and R at the 7 position. Keywords: crystal structure; indole alkaloid; absolute configuration. CCDC reference: 1578796 1. Chemical context Supporting information: this article has supporting information at journals.iucr.org/e The Madagascar periwinkle or rosy periwinkle (Catharanthus roseus L. G. Don), a member of the family Apocynaceae, is one of the most intensively studied medicinal plants (Sotto- mayor et al., 1998; Sreevalli et al., 2004). Aerial parts of the plant contain between 0.2 and 1% of a mixture of more than 120 alkaloids (van Der Heijden et al., 2004). -

Medicinal Uses, Phytochemistry and Pharmacology of Picralima Nitida
Asian Pacific Journal of Tropical Medicine (2014)1-8 1 Contents lists available at ScienceDirect Asian Pacific Journal of Tropical Medicine journal homepage:www.elsevier.com/locate/apjtm Document heading doi: Medicinal uses, phytochemistry and pharmacology of Picralima nitida (Apocynaceae) in tropical diseases: A review Osayemwenre Erharuyi1, Abiodun Falodun1,2*, Peter Langer1 1Institute of Chemistry, University of Rostock, Albert-Einstein-Str. 3A, 18059 Rostock, Germany 2Department of Pharmacognosy, School of Pharmacy, University of Mississippi, 38655 Oxford, Mississippi, USA ARTICLE INFO ABSTRACT Article history: Picralima nitida Durand and Hook, (fam. Apocynaceae) is a West African plant with varied Received 10 October 2013 applications in African folk medicine. Various parts of the plant have been employed Received in revised form 15 November 2013 ethnomedicinally as remedy for fever, hypertension, jaundice, dysmenorrheal, gastrointestinal Accepted 15 December 2013 disorders and malaria. In order to reveal its full pharmacological and therapeutic potentials, Available online 20 January 2014 the present review focuses on the current medicinal uses, phytochemistry, pharmacological and toxicological activities of this species. Literature survey on scientific journals, books as well Keywords: as electronic sources have shown the isolation of alkaloids, tannins, polyphenols and steroids Picralima nitida from different parts of the plant, pharmacological studies revealed that the extract or isolated Apocynaceae compounds from this species -

Diversity of the Mountain Flora of Central Asia with Emphasis on Alkaloid-Producing Plants
diversity Review Diversity of the Mountain Flora of Central Asia with Emphasis on Alkaloid-Producing Plants Karimjan Tayjanov 1, Nilufar Z. Mamadalieva 1,* and Michael Wink 2 1 Institute of the Chemistry of Plant Substances, Academy of Sciences, Mirzo Ulugbek str. 77, 100170 Tashkent, Uzbekistan; [email protected] 2 Institute of Pharmacy and Molecular Biotechnology, Heidelberg University, Im Neuenheimer Feld 364, 69120 Heidelberg, Germany; [email protected] * Correspondence: [email protected]; Tel.: +9-987-126-25913 Academic Editor: Ipek Kurtboke Received: 22 November 2016; Accepted: 13 February 2017; Published: 17 February 2017 Abstract: The mountains of Central Asia with 70 large and small mountain ranges represent species-rich plant biodiversity hotspots. Major mountains include Saur, Tarbagatai, Dzungarian Alatau, Tien Shan, Pamir-Alai and Kopet Dag. Because a range of altitudinal belts exists, the region is characterized by high biological diversity at ecosystem, species and population levels. In addition, the contact between Asian and Mediterranean flora in Central Asia has created unique plant communities. More than 8100 plant species have been recorded for the territory of Central Asia; about 5000–6000 of them grow in the mountains. The aim of this review is to summarize all the available data from 1930 to date on alkaloid-containing plants of the Central Asian mountains. In Saur 301 of a total of 661 species, in Tarbagatai 487 out of 1195, in Dzungarian Alatau 699 out of 1080, in Tien Shan 1177 out of 3251, in Pamir-Alai 1165 out of 3422 and in Kopet Dag 438 out of 1942 species produce alkaloids. The review also tabulates the individual alkaloids which were detected in the plants from the Central Asian mountains. -

Deploying Microbial Synthesis for Halogenating and Diversifying Medicinal Alkaloid Scaffolds
Downloaded from orbit.dtu.dk on: Sep 28, 2021 Deploying Microbial Synthesis for Halogenating and Diversifying Medicinal Alkaloid Scaffolds Bradley, Samuel Alan; Zhang, Jie; Jensen, Michael Krogh Published in: Frontiers in Bioengineering and Biotechnology Link to article, DOI: 10.3389/fbioe.2020.594126 Publication date: 2020 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Bradley, S. A., Zhang, J., & Jensen, M. K. (2020). Deploying Microbial Synthesis for Halogenating and Diversifying Medicinal Alkaloid Scaffolds. Frontiers in Bioengineering and Biotechnology, 8, [594126]. https://doi.org/10.3389/fbioe.2020.594126 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. fbioe-08-594126 October 19, 2020 Time: 19:15 # 1 REVIEW published: 23 October 2020 doi: 10.3389/fbioe.2020.594126 Deploying Microbial Synthesis for Halogenating and Diversifying Medicinal Alkaloid Scaffolds Samuel A. Bradley, Jie Zhang and Michael K. -

Solution of the Multistep Pathway for Assembly of Corynanthean, Strychnos, Iboga, and Aspidosperma Monoterpenoid Indole Alkaloids from 19E-Geissoschizine
Solution of the multistep pathway for assembly of corynanthean, strychnos, iboga, and aspidosperma monoterpenoid indole alkaloids from 19E-geissoschizine Yang Qua, Michael E. A. M. Eassona,1, Razvan Simionescub, Josef Hajicekb,2, Antje M. K. Thamma,3, Vonny Salima,4, and Vincenzo De Lucaa,5 aDepartment of Biological Sciences, Brock University, St. Catharines, ON L2S 3A1, Canada; and bDepartment of Chemistry, Brock University, St. Catharines, ON L2S 3A1, Canada Edited by Jerrold Meinwald, Cornell University, Ithaca, NY, and approved February 11, 2018 (received for review November 16, 2017) Monoterpenoid indole alkaloids (MIAs) possess a diversity of for the formation of tabersonine or catharanthine from 19E- alkaloid skeletons whose biosynthesis is poorly understood. A geissoschizine. Gene discovery involved a combination of bio- bioinformatic search of candidate genes, combined with their informatics and virus-induced gene silencing (VIGS) to identify virus-induced gene silencing, targeted MIA profiling and in vitro/ candidate genes and functional expression of the selected genes in vivo pathway reconstitution identified and functionally charac- by in vitro/in vivo reconstitution of the pathway. A series of terized six genes as well as a seventh enzyme reaction required for highly unstable intermediates that rearrange to other important the conversion of 19E-geissoschizine to tabersonine and catharan- MIA precursors when not used by appropriate enzymes reveals thine. The involvement of pathway intermediates in the formation the plasticity of MIA formation and how this fundamental of four MIA skeletons is described, and the role of stemmadenine- property led to diverse MIA structures found in nature. O-acetylation in providing necessary reactive substrates for the formation of iboga and aspidosperma MIAs is described. -

Total Synthesis of the Bridged Indole Alkaloid Apparicine
pubs.acs.org/joc Total Synthesis of the Bridged Indole Alkaloid Apparicine M.-Lluı¨ sa Bennasar,* Ester Zulaica, Daniel Sole, Tomas Roca, Davinia Garcı´ a-Dı´ az, and Sandra Alonso Laboratory of Organic Chemistry, Faculty of Pharmacy, and Institut de Biomedicina (IBUB), University of Barcelona, Barcelona 08028, Spain [email protected] Received September 15, 2009 An indole-templated ring-closing metathesis or a 2-indolylacyl radical cyclization constitute the central steps of two alternative approaches developed to assemble the tricyclic ABC substructure of the indole alkaloid apparicine. From this key intermediate, an intramolecular vinyl halide Heck reaction accomplished the closure of the strained 1-azabicyclo[4.2.2]decane framework of the alkaloid with concomitant incorporation of the exocyclic alkylidene substituents. Introduction Apparicine (Figure 1) is a fairly widespread monoterpenoid indole alkaloid, first isolated from Aspidosperma dasycarpon more than 40 years ago.1,2 Its structural elucidation,2 carried out by chemical degradation and early spectroscopic techni- ques, revealed a particular skeleton with a bridged 1-azabi- cyclo[4.2.2]decane framework fused to the indole ring and two exocyclic alkylidene (16-methylene and 20E-ethylidene) sub- Publication Date (Web): October 14, 2009 | doi: 10.1021/jo901986v stituents.3 Thesamearrangementwasalsofoundinvallesa- mine4 and later in a small number of alkaloids, including 16(S)- 5 6 Downloaded by UNIV OF BARCELONA on October 20, 2009 | http://pubs.acs.org hydroxy-16,22-dihydroapparicine or ervaticine, which differ from apparicine in the substitution at C-16.7 The apparicine alkaloids are biogenetically defined by the presence of only one carbon (C-6) connecting the indole 3- position and the aliphatic nitrogen, which is the result of the C-5 (1) Gilbert, B.; Duarte, A. -

Exploring Anti-Hyperglycemic Potential of Alkaloid Compounds from Catharanthus Roseus G
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ly et al. World Journal of Pharmacy and Pharmaceutical Sciences SJIF Impact Factor 6.647 Volume 6, Issue 8, 82-110 Research Article ISSN 2278 – 4357 EXPLORING ANTI-HYPERGLYCEMIC POTENTIAL OF ALKALOID COMPOUNDS FROM CATHARANTHUS ROSEUS G. DON (L) AGAINST VARIOUS TYPE II DIABETES TARGETS BY IN SILICO VIRTUAL SCREENING Thanh-Hoang Nguyen-Vo1,2, Dat Nguyen1, Phuc H. Le1 and Ly Le1* 1School of Biotechnology, International University - VNU, HCMC, Vietnam. 2Institute for Computational Science, Ton Duc Thang University, HCMC, Vietnam. GRAPHICAL ABSTRACT Article Received on 31 May 2017, Revised on 20 June 2017, Accepted on 11 July 2017 DOI: 10.20959/wjpps20178-9647 *Corresponding Author Ly Le School of Biotechnology, International University - VNU, HCMC, Vietnam. ABSTRACT Background: Catharanthus roseus (L.) G. Don (C. roseus) is a prominent anticancer herb having high alkaloid content. Besides known anticancer effect, this plant has been also traditionally used as an effective anti-diabetic treatment in many Asian countries, such as India, Malaysia, and Vietnam. Some in vivo and in vitro studies on its compounds and fractional extracts have confirmed its strong anti-hyperglycemia. This fact raises a question whether alkaloid compounds probably play any function in inhibitory activity on proteins related to Type 2 Diabetes Mellitus (T2DM). This study, therefore, was conducted not only to assess the role of alkaloid compounds in glucoregulatory pathway but also investigate other chemical groups treating T2DM besides known ones such as polyphenols and terpenoids. Objective: The study aims to calculate the binding affinity of alkaloids compounds of C. roseus toward four target proteins including 11β-HSD1, PTP1B, α-glucosidase and DPPIV www.wjpps.com Vol 6, Issue 8, 2017. -

Partial Purification and Properties of S-Adenosylmethionine. (R), (S
Planta Journal of Medicinal Plant Research medica Organ der Editor-in-Chief A. Baerheim-Svendsen, E. Hecker, Heidelberg J. M. Rowson, Mablethorpe Gesellschaft für E. Reinhard, Tübingen Leiden R. Hegnauer, Leiden M. v. Schantz, Helsinki Arzneipflanzen• Pharmazeutisches Institut H. Böhm, Halle W. Herz, Tallahassee K. F. Sewing, Hannover forschung Auf der Morgenstelle 8 F. Bohlmann, Berlin K. Hostettmann, Lausanne E. J. Shellard, London D-7400 Tübingen A. Cave, Chatenay-Malabry H. Inouye, Kyoto S. Shibata, Tokyo P. Delaveau, Paris M. A. Iyengar, Manipal Ch. Tamm, Basel Editorial Board Ding Guang-sheng, F. Kaiser, Mannheim W. S. Woo, Seoul H. P. T. Ammon, Tübingen Shanghai F. H. Kemper, Münster Xiao Pei-gen, Beijing W. Barz, Münster C. -J. Estler, Erlangen W. R. Kukovetz, Graz E. Reinhard, Tübingen N. Farnsworth, Chicago J. Lemli, Leuven •O. Sticher, Zürich H. Floss, Columbus Liang Xiao-tian, Beijing H. Wagner, München H. Friedrich, Münster M. Lounasmaa, Helsinki M. H. Zenk, München D. Fritz, Weihenstephan M. Luckner, Halle A. G. Gonzalez, La Laguna J. Lutomski, Poznan Advisory Board O. R. Gottlieb, Sao Paulo H. Menßen, Köln N. Anand, Lucknow E. Graf, Köln E. Noack, Düsseldorf R. Anton, Strasbourg H. Haas, Mannheim J. D. Phillipson, London Contents Volume 49,1983 ISSN 0032-0943 (f) Hippokrates Hippokrates Verlag Stuttgart II Contents 49,1983 Atta-ur-Rahman, Bashir, M.\ Isolation of New Alkaloids Cannabinoids in Phelipaea ramosa, a Parasite of Canna• from Catharanthus roseus 124 bis sativa) 250 Atta-ur-Rahman, Nisa, M., Farhi, S.: Isolation of Moenjod- aramine from Buxus papilosa 126 Galun, £., Aviv, D., Dantes, A., Freeman, A. -

Indole Alkaloids from Cell Suspension Cultures of Stemmadenia
Indole Alkaloids from Cell Suspension Cultures of Stemmadenia tomentosa and Voacanga africana Joachim Stöckigt, Karl-Heinz Pawelka, Ana Rother*, and Brigitte Deus, Institut für Pharmazeutische Biologie, Karlstr. 29, Universität München, D-8000 München 2, Bundesrepublik Deutschland Z. Naturforsch. 37 c, 857-860 (1982); received June 3, 1982 Stemmadenia tomentosa var. palmeri, Voacanga africana, Apocynaceae, Cell Suspension Cultures, Indole Alkaloids Cell suspension cultures of Stemmadenia tomentosa synthesized under normal growth condition the eight major indole alkaloids: (-)-tabersonine, (—)-minovincinine, (+)-conoflorine (voaphyl- line), condylocarpine, (+)-tubotaiwine (dihydrocondylocarpine), (-)-norfluorocurarine (vin- canine), (-)-vinervine, and (—)-coronaridine. These alkaloids consist of the three different types, Aspidosperma, Strychnos and Iboga. In contrast, cultures of Voacanga africana produced mainly one alkaloid group (Aspidosperma-type) represented by (—)-tabersonine, lochnericine and (—)- minovincinine. Therefore this cell culture seems to be qualified for investigation concerning the biosynthesis of Aspidosperma alkaloids. Introduction Stemmadenia cells produced indole alkaloids of the Strychnos-, Iboga- and Aspidosperma-type, Whereas the pattern of monoterpenoid indole whereas only alkaloids of the Aspidosperma group alkaloids in differentiated Stemmadenia and Voa have been isolated as major products from Voa canga species have been widely studied [1-3], the canga cells in culture. In view of the alkaloid com formation of these secondary products in cell sus position of Stemmadenia and Voacanga described pension cultures has not been described up to now. here, it seems that both cultures represent a good In the course of a phytochemical screening of cul tool for investigating the cell-free biosynthesis of tured cells of the Apocynaceae family [4, 5], we alkaloids originating by a pathway other than the determined here the alkaloid composition in well known leading to the Corynanthe type [8, 9].