Namenverzeichnis. Index of Names. Index Des Auteurs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

M.Sc. CHEMISTRY
M.Sc. CHEMISTRY ANALYTICAL CHEMISTRY SPECIALISATION SYLLABUS OF III & IV SEMESTERS REVISED AS PER NEW (CB) SYLLABUS FOR STUDENTS ADMITTED FROM THE YEAR 2016 ONWARDS M.Sc. CHEMISTRY (ANALYTICAL CHEMISTRY SPECIALISATION) Syllabus for III and IV Semesters (for the batches admitted in academic year 2016 & later under CBCS pattern) [Under Restructured CBCS Scheme] Grand total marks and credits (all 4 semesters) 2400 marks – 96 credits (Approved in the P.G. BOS meeting held on 01-07-2017) Semester - III (ANALYTICAL CHEMISTRY) [Under CBCS Scheme] (for the batches admitted in academic year 2016 & later under CBCS pattern) Hrs/week Internal assessment Semester exam Total Credits CH(AC)301T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)302T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)303T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)304T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)351P (LAB-I) 9 100 marks 4 CH(AC)352P (LAB-II) 9 100 marks 4 Total 600 marks 24 Semester - IV (ANALYTICAL CHEMISTRY) Hrs/week Internal assessment Semester exam Total Credits CH(AC)401T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)402T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)403T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)404T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)451P (LAB-I) 9 100 marks 4 CH(AC)452P (LAB-II) 9 100 marks 4 Total 600 marks 24 Grand total marks and credits (all 4 semesters) 2400 marks - 96 credits M.Sc.ANALYTICAL CHEMISTRY Semester III Paper I :CH (AC) 301T: CORE : Sampling, Data handling, Classical and Atomic spectral methods -
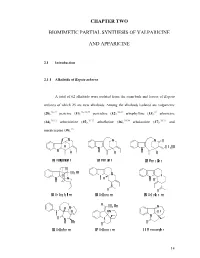
Chapter Two Biomimetic Partial Synthesis Of
CHAPTER TWO BIOMIMETIC PARTIAL SYNTHESIS OF VALPARICINE AND APPARICINE 2.1 Introduction 2.1.1 Alkaloids of Kopsia arborea A total of 62 alkaloids were isolated from the stem-bark and leaves of Kopsia arborea of which 25 are new alkaloids. Among the alkaloids isolated are valparicine (28),26,27 pericine (31),26,28,29 pericidine (32),30,31 arbophylline (33),32 arboricine (34),30,33 arboricinine (35),30,33 arboflorine (36),30,34 arboloscine (37),30,31 and mersicarpine (38).35 14 The compounds of interest to this research are valparicine (28) and pericine (31). Valparicine (28) was isolated from the stem-bark extract of K. arborea in trace amount. It represents the first member of the pericine-type alkaloids, characterized by a 16-22 exocyclic double bond, in which bond-formation has occurred between C-3 and C-7.26 Preliminary tests indicated that valparicine (28) showed strong cytotoxic effects −1 against human KB cells (IC50 < 5 μgmL ). Valparicine (28) was obtained as a colorless oil, with [α]D −40 (c 0.22, CHCl3). The UV spectrum (228 and 297 nm) indicated the presence of an unsubstituted indolenine chromophore. The EIMS of 28 showed a molecular ion at m/z 276, which 13 analyzed for C19H20N2, differing from pericine (31) by loss of two hydrogens. The C NMR spectrum gave a total of 19 carbon resonances (one methyl, five methylenes, seven methines, and six quaternary carbons) in agreement with the molecular formula. In addition to the six carbon resonances readily attributable to the aromatic moiety, and the imine resonance at δC 186.4, two other downfield quaternary resonances were observed at δC 139.2 and 144.6. -

United States Patent (19) 11 Patent Number: 5,627,195 Hu 45 Date of Patent: May 6, 1997
USOO5627195A United States Patent (19) 11 Patent Number: 5,627,195 Hu 45 Date of Patent: May 6, 1997 54 TREATMENT FOR OCULAR Ferrante, et al., Tetrandrine, a Plant Alkaloid, Inhibits the NFLAMMATON Production of Tumour Necrosis Factor-Alpha (Cachectin) by Human Monocytes, Clin, exp. Immunol. 80:232–235 75 Inventor: Shixing Hu, Cambridge, Mass. (1990). Kondo, et al., Inhibitory Effect of Bisbenzylisoquinoline 73) Assignee: Massachusetts Eye and Ear Alkaloids on the Quick Death of Mice Treated with BCG/ Infirmary, Boston, Mass. LPS, Chem. Pharm. bull. 38(10):287-2889 (1990). Ph.D. dissertation of Shixing Hu, Sun Yat-Sen University of 21 Appl. No.: 420,244 Medical Sciences, Guang Zhou, Guang Dong China, 1989. 22 Filed: Apr. 11, 1995 Seow, et al. In Vitro Immunosuppressive Properties of Teh Plant Alkaloid Tetrandrine. Int. Archs Allergy appl. Immun. [51] Int. Cl. ... A61K 31/445 85:410-415 (1988). 52 514/321: 514/912 Rao. et al., Modulation of Lens-Induced Uvetis by Super 58 Field of Search ...................................... 54/321, 912 oxide Dismutase, Opthlalmic Res. 18:41-46 (1986). Abal et al., Clinical Evaluation of Berberine in Mycotic 56 References Cited Infections, Ind. J. Ophthalm. 34:91-2 (1986). FOREIGN PATENT DOCUMENTS Mohan, et al. Berberine: An Indigenous Drug in Experi mental Herpetic Uveitis. Ind.J. Ophthalm. 31:65-68 (1983). 46-21396 6/1971 Japan. Yao Hsueh Tung Pao 1983; 18:31-36 The Natural Sources 3-44323 2/1991 Japan. and Biological Activities of Bisbenzylisoquinoline (BBI) 4–99723 3/1992 Japan. Alkaloids. OTHER PUBLICATIONS Babbar, et al., Effect of Berberine Chloride Eye Drops on Marshall, et al. -

Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Chronic Venous Insufficiency/CVI
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Chronic Venous Insufficiency/CVI Chemical Activity Count (+)-AROMOLINE 1 (+)-CATECHIN 5 (+)-GALLOCATECHIN 1 (+)-HERNANDEZINE 1 (+)-PRAERUPTORUM-A 1 (+)-SYRINGARESINOL 1 (+)-SYRINGARESINOL-DI-O-BETA-D-GLUCOSIDE 1 (-)-ACETOXYCOLLININ 1 (-)-APOGLAZIOVINE 1 (-)-BISPARTHENOLIDINE 1 (-)-BORNYL-CAFFEATE 1 (-)-BORNYL-FERULATE 1 (-)-BORNYL-P-COUMARATE 1 (-)-CANADINE 1 (-)-EPICATECHIN 4 (-)-EPICATECHIN-3-O-GALLATE 1 (-)-EPIGALLOCATECHIN 1 (-)-EPIGALLOCATECHIN-3-O-GALLATE 2 (-)-EPIGALLOCATECHIN-GALLATE 3 (-)-HYDROXYJASMONIC-ACID 1 (-)-N-(1'-DEOXY-1'-D-FRUCTOPYRANOSYL)-S-ALLYL-L-CYSTEINE-SULFOXIDE 1 (1'S)-1'-ACETOXYCHAVICOL-ACETATE 1 (2R)-(12Z,15Z)-2-HYDROXY-4-OXOHENEICOSA-12,15-DIEN-1-YL-ACETATE 1 (7R,10R)-CAROTA-1,4-DIENALDEHYDE 1 (E)-4-(3',4'-DIMETHOXYPHENYL)-BUT-3-EN-OL 1 1,2,6-TRI-O-GALLOYL-BETA-D-GLUCOSE 1 1,7-BIS(3,4-DIHYDROXYPHENYL)HEPTA-4E,6E-DIEN-3-ONE 1 Chemical Activity Count 1,7-BIS(4-HYDROXY-3-METHOXYPHENYL)-1,6-HEPTADIEN-3,5-DIONE 1 1,8-CINEOLE 1 1-(METHYLSULFINYL)-PROPYL-METHYL-DISULFIDE 1 1-ETHYL-BETA-CARBOLINE 1 1-O-(2,3,4-TRIHYDROXY-3-METHYL)-BUTYL-6-O-FERULOYL-BETA-D-GLUCOPYRANOSIDE 1 10-ACETOXY-8-HYDROXY-9-ISOBUTYLOXY-6-METHOXYTHYMOL 1 10-GINGEROL 1 12-(4'-METHOXYPHENYL)-DAURICINE 1 12-METHOXYDIHYDROCOSTULONIDE 1 13',II8-BIAPIGENIN 1 13-HYDROXYLUPANINE 1 14-ACETOXYCEDROL 1 14-O-ACETYL-ACOVENIDOSE-C 1 16-HYDROXY-4,4,10,13-TETRAMETHYL-17-(4-METHYL-PENTYL)-HEXADECAHYDRO- 1 CYCLOPENTA[A]PHENANTHREN-3-ONE 2,3,7-TRIHYDROXY-5-(3,4-DIHYDROXY-E-STYRYL)-6,7,8,9-TETRAHYDRO-5H- -

Analytical Reference Standards
Cerilliant Quality ISO GUIDE 34 ISO/IEC 17025 ISO 90 01:2 00 8 GM P/ GL P Analytical Reference Standards 2 011 Analytical Reference Standards 20 811 PALOMA DRIVE, SUITE A, ROUND ROCK, TEXAS 78665, USA 11 PHONE 800/848-7837 | 512/238-9974 | FAX 800/654-1458 | 512/238-9129 | www.cerilliant.com company overview about cerilliant Cerilliant is an ISO Guide 34 and ISO 17025 accredited company dedicated to producing and providing high quality Certified Reference Standards and Certified Spiking SolutionsTM. We serve a diverse group of customers including private and public laboratories, research institutes, instrument manufacturers and pharmaceutical concerns – organizations that require materials of the highest quality, whether they’re conducing clinical or forensic testing, environmental analysis, pharmaceutical research, or developing new testing equipment. But we do more than just conduct science on their behalf. We make science smarter. Our team of experts includes numerous PhDs and advance-degreed specialists in science, manufacturing, and quality control, all of whom have a passion for the work they do, thrive in our collaborative atmosphere which values innovative thinking, and approach each day committed to delivering products and service second to none. At Cerilliant, we believe good chemistry is more than just a process in the lab. It’s also about creating partnerships that anticipate the needs of our clients and provide the catalyst for their success. to place an order or for customer service WEBSITE: www.cerilliant.com E-MAIL: [email protected] PHONE (8 A.M.–5 P.M. CT): 800/848-7837 | 512/238-9974 FAX: 800/654-1458 | 512/238-9129 ADDRESS: 811 PALOMA DRIVE, SUITE A ROUND ROCK, TEXAS 78665, USA © 2010 Cerilliant Corporation. -

Studies on the Synthesis and Biosynthesis Of
STUDIES ON THE SYNTHESIS AND BIOSYNTHESIS OF INDOLE ALKALOIDS BY GEORGE BOHN FULLER B.A. (cum laude) , Macalester College, 1969 M.Sc, The University of California, Berkeley, 19 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in the Department of CHEMISTRY We accept this thesis as conforming to the required standard /-) THE UNIVERSITY OF BRITISH COLUMBIA July, 1974 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the Head of my Department or by his representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Depa rtment The University of British Columbia Vancouver 8, Canada ABSTRACT Part A of this thesis provides a resume1 of the synthesis of various radioactively labelled forms of secodine C76) and provides an evaluation of these compounds, as well as some radioactively labelled forms of tryptophan C25), as precursors in the Biosynthesis of apparicine (81), uleine C83), guatam- buine (90) , and olivacine (88) in Aspidosperma australe. Only apparicine (81) could be shown to incorporate these precursors to a significant extent. Degradation of apparicine (81) from Aspidosperma pyricollum provided evidence for the intact incorporation of the secodine system. Part B discusses the synthesis of 16-epi-stemmadenine (161), which provides an entry into the stemmadenine system with, radioactive labels at key positions in the molecule. -

Accepted Version
Article Metabolomics identifies a biomarker revealing in vivo loss of functional ß-cell mass before diabetes onset LI, Lingzi, et al. Abstract Identification of pre-diabetic individuals with decreased functional ß-cell mass is essential for the prevention of diabetes. However, in vivo detection of early asymptomatic ß-cell defect remains unsuccessful. Metabolomics emerged as a powerful tool in providing read-outs of early disease states before clinical manifestation. We aimed at identifying novel plasma biomarkers for loss of functional ß-cell mass in the asymptomatic pre-diabetic stage. Non-targeted and targeted metabolomics were applied on both lean ß-Phb2-/- mice (ß-cell-specific prohibitin-2 knockout) and obese db/db mice (leptin receptor mutant), two distinct mouse models requiring neither chemical nor diet treatments to induce spontaneous decline of functional ß-cell mass promoting progressive diabetes development. Non-targeted metabolomics on ß-Phb2-/- mice identified 48 and 82 significantly affected metabolites in liver and plasma, respectively. Machine learning analysis pointed to deoxyhexose sugars consistently reduced at the asymptomatic pre-diabetic stage, including in db/db mice, showing strong correlation with the gradual loss of ß-cells. [...] Reference LI, Lingzi, et al. Metabolomics identifies a biomarker revealing in vivo loss of functional ß-cell mass before diabetes onset. Diabetes, 2019, vol. 68, no. 12, p. 2272-2286 PMID : 31537525 DOI : 10.2337/db19-0131 Available at: http://archive-ouverte.unige.ch/unige:126176 -

Total Synthesis of the Bridged Indole Alkaloid Apparicine
pubs.acs.org/joc Total Synthesis of the Bridged Indole Alkaloid Apparicine M.-Lluı¨ sa Bennasar,* Ester Zulaica, Daniel Sole, Tomas Roca, Davinia Garcı´ a-Dı´ az, and Sandra Alonso Laboratory of Organic Chemistry, Faculty of Pharmacy, and Institut de Biomedicina (IBUB), University of Barcelona, Barcelona 08028, Spain [email protected] Received September 15, 2009 An indole-templated ring-closing metathesis or a 2-indolylacyl radical cyclization constitute the central steps of two alternative approaches developed to assemble the tricyclic ABC substructure of the indole alkaloid apparicine. From this key intermediate, an intramolecular vinyl halide Heck reaction accomplished the closure of the strained 1-azabicyclo[4.2.2]decane framework of the alkaloid with concomitant incorporation of the exocyclic alkylidene substituents. Introduction Apparicine (Figure 1) is a fairly widespread monoterpenoid indole alkaloid, first isolated from Aspidosperma dasycarpon more than 40 years ago.1,2 Its structural elucidation,2 carried out by chemical degradation and early spectroscopic techni- ques, revealed a particular skeleton with a bridged 1-azabi- cyclo[4.2.2]decane framework fused to the indole ring and two exocyclic alkylidene (16-methylene and 20E-ethylidene) sub- Publication Date (Web): October 14, 2009 | doi: 10.1021/jo901986v stituents.3 Thesamearrangementwasalsofoundinvallesa- mine4 and later in a small number of alkaloids, including 16(S)- 5 6 Downloaded by UNIV OF BARCELONA on October 20, 2009 | http://pubs.acs.org hydroxy-16,22-dihydroapparicine or ervaticine, which differ from apparicine in the substitution at C-16.7 The apparicine alkaloids are biogenetically defined by the presence of only one carbon (C-6) connecting the indole 3- position and the aliphatic nitrogen, which is the result of the C-5 (1) Gilbert, B.; Duarte, A. -

Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Tinnitus
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Tinnitus Chemical Activity Count (+)-ALPHA-VINIFERIN 1 (+)-AROMOLINE 1 (+)-BORNYL-ISOVALERATE 1 (+)-CATECHIN 1 (+)-EUDESMA-4(14),7(11)-DIENE-3-ONE 1 (+)-HERNANDEZINE 2 (+)-ISOLARICIRESINOL 1 (+)-NORTRACHELOGENIN 1 (+)-PSEUDOEPHEDRINE 1 (+)-SYRINGARESINOL-DI-O-BETA-D-GLUCOSIDE 1 (+)-T-CADINOL 1 (-)-16,17-DIHYDROXY-16BETA-KAURAN-19-OIC 1 (-)-ALPHA-BISABOLOL 1 (-)-ANABASINE 1 (-)-APOGLAZIOVINE 1 (-)-BETONICINE 1 (-)-BORNYL-CAFFEATE 1 (-)-BORNYL-FERULATE 1 (-)-BORNYL-P-COUMARATE 1 (-)-CANADINE 1 (-)-DICENTRINE 1 (-)-EPICATECHIN 2 (-)-EPIGALLOCATECHIN-GALLATE 1 (1'S)-1'-ACETOXYCHAVICOL-ACETATE 1 (E)-4-(3',4'-DIMETHOXYPHENYL)-BUT-3-EN-OL 1 1,7-BIS-(4-HYDROXYPHENYL)-1,4,6-HEPTATRIEN-3-ONE 1 1,8-CINEOLE 4 Chemical Activity Count 1-ETHYL-BETA-CARBOLINE 2 10-ACETOXY-8-HYDROXY-9-ISOBUTYLOXY-6-METHOXYTHYMOL 1 10-DEHYDROGINGERDIONE 1 10-GINGERDIONE 1 12-(4'-METHOXYPHENYL)-DAURICINE 1 12-METHOXYDIHYDROCOSTULONIDE 1 13',II8-BIAPIGENIN 1 13-HYDROXYLUPANINE 1 13-OXYINGENOL-ESTER 1 16,17-DIHYDROXY-16BETA-KAURAN-19-OIC 1 16-HYDROXY-4,4,10,13-TETRAMETHYL-17-(4-METHYL-PENTYL)-HEXADECAHYDRO- 1 CYCLOPENTA[A]PHENANTHREN-3-ONE 16-HYDROXYINGENOL-ESTER 1 2'-O-GLYCOSYLVITEXIN 1 2-BETA,3BETA-27-TRIHYDROXYOLEAN-12-ENE-23,28-DICARBOXYLIC-ACID 1 2-METHYLBUT-3-ENE-2-OL 2 2-VINYL-4H-1,3-DITHIIN 1 20-DEOXYINGENOL-ESTER 1 22BETA-ESCIN 1 24-METHYLENE-CYCLOARTANOL 2 3,3'-DIMETHYLELLAGIC-ACID 1 3,4-DIMETHOXYTOLUENE 2 3,4-METHYLENE-DIOXYCINNAMIC-ACID-BORNYL-ESTER 1 3,4-SECOTRITERPENE-ACID-20-EPI-KOETJAPIC-ACID -

Partial Purification and Properties of S-Adenosylmethionine. (R), (S
Planta Journal of Medicinal Plant Research medica Organ der Editor-in-Chief A. Baerheim-Svendsen, E. Hecker, Heidelberg J. M. Rowson, Mablethorpe Gesellschaft für E. Reinhard, Tübingen Leiden R. Hegnauer, Leiden M. v. Schantz, Helsinki Arzneipflanzen• Pharmazeutisches Institut H. Böhm, Halle W. Herz, Tallahassee K. F. Sewing, Hannover forschung Auf der Morgenstelle 8 F. Bohlmann, Berlin K. Hostettmann, Lausanne E. J. Shellard, London D-7400 Tübingen A. Cave, Chatenay-Malabry H. Inouye, Kyoto S. Shibata, Tokyo P. Delaveau, Paris M. A. Iyengar, Manipal Ch. Tamm, Basel Editorial Board Ding Guang-sheng, F. Kaiser, Mannheim W. S. Woo, Seoul H. P. T. Ammon, Tübingen Shanghai F. H. Kemper, Münster Xiao Pei-gen, Beijing W. Barz, Münster C. -J. Estler, Erlangen W. R. Kukovetz, Graz E. Reinhard, Tübingen N. Farnsworth, Chicago J. Lemli, Leuven •O. Sticher, Zürich H. Floss, Columbus Liang Xiao-tian, Beijing H. Wagner, München H. Friedrich, Münster M. Lounasmaa, Helsinki M. H. Zenk, München D. Fritz, Weihenstephan M. Luckner, Halle A. G. Gonzalez, La Laguna J. Lutomski, Poznan Advisory Board O. R. Gottlieb, Sao Paulo H. Menßen, Köln N. Anand, Lucknow E. Graf, Köln E. Noack, Düsseldorf R. Anton, Strasbourg H. Haas, Mannheim J. D. Phillipson, London Contents Volume 49,1983 ISSN 0032-0943 (f) Hippokrates Hippokrates Verlag Stuttgart II Contents 49,1983 Atta-ur-Rahman, Bashir, M.\ Isolation of New Alkaloids Cannabinoids in Phelipaea ramosa, a Parasite of Canna• from Catharanthus roseus 124 bis sativa) 250 Atta-ur-Rahman, Nisa, M., Farhi, S.: Isolation of Moenjod- aramine from Buxus papilosa 126 Galun, £., Aviv, D., Dantes, A., Freeman, A. -

Methoxy-3-Veratryl-5,6-Dihydrobenzoglyoxalocoline T
HFTEROCYCLES. Vol 30, No I, 1990 PAPERS OF PROFESSOR TETSUJI KAMETANI 1 Studies on the Synthesis of Emetine and Related Compounds. VI. A Syn thesis of 4'. 5'-Methylenedioxy-6-[B-6". 7"-methylenedioxy-3"-methyl-py- tetrahydroisoquinolyl-I"] -ethyl]-4-methyl-3,4,5,6,7, B-hexahydro- ( 1 2 : 1.2-benzoquinolizine) S . Sugasawa and T . Kametani [Yakugaku Zasshi. 65, 372 - 373 (194511 2 Syntheses of Imidazoisoquinoline Derivatives. I. Synthesis of 9.10- Dimethoxy- 3- (p-aminophenyl)- -5.6-dihydrohenzoglyoxalocoline T . Kametani and H. Iida [ Yakugaku Zasshi, 70, 258 - 261 (19501 1 3 Syntheses of Imidazoisoquinoline Derivatives. 11. Synthesis of 9.10-Di- methoxy-3-veratryl-5,6-dihydrobenzoglyoxalocoline T. Kametani and H. Iwakata [ Yakugaku Zasshi, 70, 261 - 263 (1950) 1 4 Syntheses of Imidazoisoquinoline Derivatives. 111. Synthesis of 9.10-Di- methoxy- 3-safryl- 5.6-dihydrobenzoglyoxalocoline T . Kametani and H. Iwakata [Yakugaku Zasshi, 70. 263 - 265 (195011 5 Syntheses of Papaverine Derivatives. I. Synthesis of 1-(3'-Methoxy-4'- hydroxyphenyl) -3-methyl-6.7-methylenedioxyisoquinoline T. Kametani [ Yakugaku Zasshi, 71. 322 - 324 (1951) I 6 Syntheses of Imidazoisoquinoline Derivatives. IV. Syntheses of 9,lO- Methylenedioxy- 3- (4'-nitrophenyl) - 5-methyl- 5.6-dihydrobenzoglyoxalocoline. 9,lO-Methylenedioxy-3- (3', 4'-dimethoxypheny1)-5-methylbenzoglyoxalocoline and 9,lO-Methylenedioxy-3-( 3'. 4'-methylenedioxyphenyl]-5-methyl-5,6- dihydrobenzoglyo::alocoline T. Kametani, H. Iida, and H. Iwakata [ Yakugaku Zasshi. 71. 325- 329 (195111 Studies on the Syntheses of Aminoisoquinoline Derivatives. I. Saponification of Benzyl 1-Phenyl-6.7-dimethoxyisoquinoly1-3-carbamate T. Kametani [ Yakugaku Zasshi, 71. 329 - 331 (1951) I Studies on the Syntheses of Aminoisoquinoline Derivatives. -

UC Riverside UC Riverside Previously Published Works
UC Riverside UC Riverside Previously Published Works Title The biology and total syntheses of bisbenzylisoquinoline alkaloids. Permalink https://escholarship.org/uc/item/67s758hb Journal Organic & biomolecular chemistry, 19(35) ISSN 1477-0520 Authors Nguyen, Viviene K Kou, Kevin GM Publication Date 2021-09-15 DOI 10.1039/d1ob00812a Peer reviewed eScholarship.org Powered by the California Digital Library University of California Please do not adjust margins REVIEW The biology and total syntheses of bisbenzylisoquinoline alkaloids Viviene K. Nguyen, Kevin. G. M. Kou* Received 00th January 20xx, Accepted 00th January 20xx DOI: 10.1039/x0xx00000x This mini-review provides a concise overview of the biosynthetic pathway and pharmacology of the bisbenzylisoquinoline alkaloid (bisBIA) natural poducts. Additional emphasis is given to the methodologies in the total syntheses of both the simpler acyclic diaryl ether dimers and their macrocyclic counterparts bearing two diaryl ether linkages. 1. Introduction and classification The benzylisoquinoline alkaloids (BIAs) represent a family of over 2500 plant natural products that have been used for centuries as analgesics and wound disinfectants.1 Some of the active and biosynthetically-related members, have been exploited in modern medicine, for example, morphine for pain, 2 colchicine for gout, and noscapine for cough and cancer. In Figure 1. Coclaurine (1 and 2) and/or reticuline (3) are the recent years, the pursuit of bisbenzylisoquinoline alkaloids biosynthetic building blocks of the majority of bisBIAs. (bisBIAs), molecules comprising two BIA motifs, is gaining traction. Isolated from the Berberidaceae, Ranunculaceae, their botanical sources as well as spectral and physical data.1-8 Lauraceae and Menispermaceae plant families, these This review highlights the biosynthesis and medicinal compounds can modulate diverse biological functions.3,4 With implications of bisBIAs.