Safety Data Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(VI) and Chromium (V) Oxide Fluorides
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1976 The chemistry of chromium (VI) and chromium (V) oxide fluorides Patrick Jay Green Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Chemistry Commons Let us know how access to this document benefits ou.y Recommended Citation Green, Patrick Jay, "The chemistry of chromium (VI) and chromium (V) oxide fluorides" (1976). Dissertations and Theses. Paper 4039. https://doi.org/10.15760/etd.5923 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. All ABSTRACT OF THE TllESIS OF Patrick Jay Green for the Master of Science in Chemistry presented April 16, 1976. Title: Chemistry of Chromium(VI) and Chromium(V) Oxide Fluorides. APPROVEO BY MEMBERS OF THE THESIS CO'"o\l TIEE: y . • Ii . ' I : • • • • • New preparative routes to chromyl fluoride were sought. It was found that chlorine ironofluoride reacts with chromium trioxide and chromyl chlo ride to produce chromyl fluoride. Attempts were ~ade to define a mechan ism for the reaction of ClF and Cr0 in light of by-products observed 3 and previous investigations. Carbonyl fluoride and chromium trioxide react to fom chro·yl fluoride and carbo:i dioxide. A mechanism was also proposed for this react10n. Chromium trioxide 11itl\ l~F6 or WF5 reacts to produce chromyl fluoride and the respective oxide tetrafluoride. 2 Sulfur hexafluoride did not react with Cr03. -
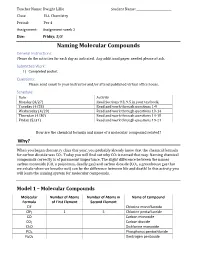
Naming Molecular Compounds General Instructions: Please Do the Activities for Each Day As Indicated
Teacher Name: Dwight Lillie Student Name: ________________________ Class: ELL Chemistry Period: Per 4 Assignment: Assignment week 2 Due: Friday, 5/8 Naming Molecular Compounds General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Submitted Work: 1) Completed packet. Questions: Please send email to your instructor and/or attend published virtual office hours. Schedule: Date Activity Monday (4/27) Read Sections 9.3, 9.5 in your textbook. Tuesday (4/28) Read and work through questions 1-9 Wednesday (4/29) Read and work through questions 10-14 Thursday (4/30) Read and work through questions 14-18 Friday (5/31) Read and work through questions 19-21 How are the chemical formula and name of a molecular compound related? Why? When you began chemistry class this year, you probably already knew that the chemical formula for carbon dioxide was CO2. Today you will find out why CO2 is named that way. Naming chemical compounds correctly is of paramount importance. The slight difference between the names carbon monoxide (CO, a poisonous, deadly gas) and carbon dioxide (CO2, a greenhouse gas that we exhale when we breathe out) can be the difference between life and death! In this activity you will learn the naming system for molecular compounds. Model 1 – Molecular Compounds Molecular Number of Atoms Number of Atoms in Name of Compound Formula of First Element Second Element ClF Chlorine monofluoride ClF5 1 5 Chlorine pentafluoride CO Carbon monoxide CO2 Carbon dioxide Cl2O Dichlorine monoxide PCl5 Phosphorus pentachloride N2O5 Dinitrogen pentoxide 1. Fill in the table to indicate the number of atoms of each type in the molecular formula. -

The Synthesis and Characterization of Novel Pentafluorosulfanyl-Containing Heterocycles and Pentafluorosulfanyldifluoromethane
Clemson University TigerPrints All Dissertations Dissertations May 2019 The yS nthesis and Characterization of Novel Pentafluorosulfanyl-Containing Heterocycles and Pentafluorosulfanyldifluoromethane Steven Paul Belina Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Recommended Citation Belina, Steven Paul, "The yS nthesis and Characterization of Novel Pentafluorosulfanyl-Containing Heterocycles and Pentafluorosulfanyldifluoromethane" (2019). All Dissertations. 2373. https://tigerprints.clemson.edu/all_dissertations/2373 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. THE SYNTHESIS AND CHARACTERIZATION OF NOVEL PENTAFLUOROSULFANYL-CONTAINING HETEROCYCLES AND PENTAFLUOROSULFANYLDIFLUOROMETHANE A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Chemistry by Steven Paul Belina May 2019 Accepted by Joseph S. Thrasher, Ph.D., Committee Chair Julia L. Brumaghim, Ph.D. R. Karl Dieter, Ph.D. Joseph W. Kolis, Ph.D. Title Page ABSTRACT Beginning in the early 1960s, scientists began to experiment with the pentafluorosulfanyl moiety. The unique properties of the functional group has attracted interest among fluorine chemists and more recently even organic chemists. These properties include high group electronegativity, high steric bulk, high lipophilicity, a highly electron withdrawing nature, and a square pyramidal geometry. However, the development and deployment of the pentafluorosulfanyl functional group has been significantly slowed. The main cause for the slow development is a lack of easily available reagents to synthesize pentafluorosulfanyl-containing compounds. Historically, the primary reagents used for synthesizing pentafluorosulfanyl-containing compounds were SF5Cl and SF5Br. -

The WMS Model
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is © the Owner Societies 2018 S-1 ELECTRONIC SUPPLEMENTARY INFORMATION OCT. 8, 2018 Extrapolation of High-Order Correlation Energies: The WMS Model Yan Zhao,a Lixue Xia,a Xiaobin Liao,a Qiu He,a Maria X. Zhao,b and Donald G. Truhlarc aState Key Laboratory of Silicate Materials for Architectures,International School of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, People’s Republic of China. bLynbrook High School, 1280 Johnson Avenue, San Jose, California 95129 cDepartment of Chemistry and Supercomputing Institute, University of Minnesota, 207 Pleasant Street S.E., Minneapolis, MN 55455-0431 TABLE OF CONTENTS Table S1: Calculated classical barrier heights (kcal/mol) for the DBH24-W4 database S-2 Table S2: RMSE of the scalar relativistic contribution in WMS (kcal/mol) S-3 Table S3: RMSE of the composite methods for the W4-17 database (kcal/mol) S-4 Table S4: Calculated Born-Oppenheimer atomization energy (kcal/mol) for the W4-17 database S-5 Table S5: Example input file for a WMS calculation: the H2O molecule S-11 Table S6: Example input file for a WMS calculation: the CH3 radical S-12 Table S7. perl scripts for performing WMS calculations S-13 S-2 Table S1: Calculated classical barrier heights (kcal/mol) for the DBH24-W4 database (The ZPE contributions are excluded.) Reactions Best Est. WMS Hydrogen Atom Transfers ! ∆E! 6.35 6.25 OH + CH4 → CH3 + H2O ! ∆E! 19.26 19.28 ! ∆E! 10.77 10.88 H + OH → O + H2 ! ∆E! 13.17 -

United States Patent Office Patented Mar
3,373,000 United States Patent Office Patented Mar. 2, 1968 2 3,373,000 monofluoride is also an economical reactant, since it is PROCESS FOR PREPARING TETRAFLUORDES conveniently prepared by the disproportionation of ele AND HEXAFLUOR DES mental chlorine in liquid hydrogen fluoride, thereby avoid James J. Pitts, West Haven, and Albert W. Jache, North ing the use of expensive elemental fluorine. The selective Haven, Conn., assignors to Olin Mathieson Chemical fluorination process of this invention is particularly Sur Corporation, a corporation of Virginia prising and unexpected in view of the prior art which No Drawing. Filed Nov. 1, 1966, Ser. No. 591,079 teaches that chlorine monofluoride adds to a wide variety 9 Claims. (Cl. 23-352) of compounds, thereby acting as a chloro-fluorinating agent. For instance, U.S. Patent 3,035,893 discloses the 10 preparation of sulfur chloride pentafluoride from Sulfur ABSTRACT OF THE DISCLOSURE tetrafluoride and chlorine monofluoride. The tetrafluorides and hexafluorides of sulfur, selenium According to the process of this invention, the tetra and tellurium are provided by reacting chlorine mono fluorides and hexafluorides of sulfur, selenium and tel fluoride with sulfur, selenium, tellurium, metal sulfides, lurium are provided by reacting chlorine monofluoride metal selenides or metal tellurides. The hexafluorides of 15 with a material selected from the group consisting of Sul tungsten, molybdenum and uranium are provided by the fur, selenium, tellurium, metal sulfides, metal selenides reaction of chlorine monofluoride with the metals, their and metal tellurides. The hexafluorides of tungsten, oxides, sulfides and salts containing the metallate anion. molybdenum and uranium are provided by reacting These tetrafluorides and hexafluorides are well-known chlorine monofluoride with a material selected from the compounds, useful as fluorinating agents, gaseous di 20 group consisting of tungsten; molybdenum, uranium; the electrics, sources of high purity metallic powders, etc. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Chlorine Trifluoride
Safetygram #39 Chlorine Trifluoride Warning: Improper storage, handling or use of chlorine trifluoride can result in serious injury and/or prop- erty damage. Use this product in accordance with the Air Products Material Safety Data Sheet (MSDS). Introduction Chlorine trifluoride (ClF3) is a toxic, corrosive, very est in the use of ClF3 for organic synthesis work reactive liquefied compressed gas packaged in increased although the material was eventually cylinders as a liquid under its own vapor pressure considered to be too reactive for practical use 2 2 of 1.55 kg/cm at 21°C (22 psia at 70°F). ClF3 is and mostly abandoned for these applications. a very useful chemical in operations requiring a Synthesis reactions proved difficult to control and high-energy fluorinating agent or incendiary mate- usually led to a wide variety of reaction by- rial, especially since it can be handled at room products that were hazardous. temperatures. However, those same factors that make it quite useful also contribute to several high Many fluorinating compounds were evaluated as hazard potentials for the product. potent oxidizers for liquid-fueled rockets in the late 1940s through the early 1950s to overcome the Air Products has been the primary manufacturer storage and handling disadvantages of liquid F2. and distributor of ClF3 in North America for the past ClF3 was first tested in the U.S. in 1948 on a liquid 35 years, and the compound represents one of the propellant rocket motor using hydrazine as the highest-reactivity products that Air Products cur- fuel. Additional testing yielded favorable results. -

Fluorine in Organic Chemistry Final Proof 7.8.2004 10:34Am Page I
Chambers: Fluorine in Organic Chemistry Final Proof 7.8.2004 10:34am page i Fluorine in Organic Chemistry Fluorine in Organic Chemistry Richard D. Chambers © 2004 Blackwell Publishing Ltd. ISBN: 978-1-405-10787-7 Chambers: Fluorine in Organic Chemistry Final Proof 7.8.2004 10:34am page iii Fluorine in Organic Chemistry Richard D. Chambers FRS Emeritus Professor of Chemistry University of Durham, UK Chambers: Fluorine in Organic Chemistry Final Proof 7.8.2004 10:34am page iv ß 2004 by Blackwell Publishing Ltd Editorial offices: Blackwell Publishing Ltd, 9600 Garsington Road, Oxford OX4 2DQ, UK Tel: þ44 (0)1865 776868 Blackwell Publishing Asia Pty Ltd, 550 Swanston Street, Carlton, Victoria 3053, Australia Tel: þ61 (0)3 8359 1011 ISBN 1-4051-0787-1 Published in the USA and Canada (only) by CRC Press LLC, 2000 Corporate Blvd., N.W., Boca Raton, FL 33431, USA Orders from the USA and Canada (only) to CRC Press LLC USA and Canada only: ISBN 0-8493-1790-8 The right of the Author to be identified as the Author of this Work has been asserted in accordance with the Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. This book contains information obtained from authentic and highly regarded sources. Reprinted material is quoted with permission, and sources are indicated. -

CHEM Safety Manual
Department of Chemistry Safety Manual October 2018 Safety Committee Department of Chemistry Hong Kong University of Science and Technology Table of Contents 1.0 Introduction 2.0 Safety Policy and Responsibility for Safety 2.1 Department Head 2.2 Department of Chemistry Safety Committee 2.3 Laboratory Supervisors 2.4 Researchers 2.5 HSEO 3.0 Information, Training, Safety Clearance, and Safety Clearance at Termination 3.1 Initial Training 3.2 Information on Hazardous Substances 3.3 Additional Safety Information 3.4 Safety Clearance at Termination 4.0 Personal Protective Equipment and Safety Engineering Controls 4.1 Eye Protection 4.2 Protective Apparel 4.3 Respirators 4.4 Laboratory Fume Cupboards 4.5 Fire Extinguishers, Safety Showers, and Eyewash Facilities 5.0 Standard Operating Procedures for Work with Hazardous Substances 5.1 Classes of Hazardous Substances 5.2 General Procedures for Work with Toxic Substances 5.3 General Procedures for Work with Flammable and Explosive Substances 6.0 Procedures for Work with Particularly Hazardous Substances 6.1 Identification and Classification of Particularly Hazardous Substances 6.2 Designated Areas 6.3 General Procedures for Work with Substances of Moderate to High Chronic or High Acute Toxicity 6.4 Additional Procedures for Work with Substances of Known High Chronic Toxicity 6.5 Specific Handling Procedures for Some Common Particularly Hazardous Substances 7.0 Proper Planning of Laboratory Work 7.1 Recognition and Assessment 7.2 Planning for the Unexpected: What Could Go Wrong? 7.3 Site Selection -

Chemical Hygiene Plan
Laboratory Safety, Chemical Hygiene Plan Occupational Exposure to Hazardous Chemicals in Laboratories and the United Nations Globally Harmonized System (GHS) 1910.1450 - Occupational Exposure to Hazardous Chemicals in Laboratories. I. Introduction II. Purpose III. Scope IV. Responsibilities V. Medical Consultations/Examinations VI. Hazard Identification VII. Training VIII. Departmental Standard Operating Procedures IX. Air Monitoring 1910 Subpart Z - Toxic and Hazardous Substances X. Laboratory Safety Guidelines 1. Housekeeping and Chemical Storage. 2. Personal Hygiene. 3. Safety Equipment. 4. Inspections. 5. Handling and Use of Flammable Chemicals. 6. Partial List of Pyrophoric Chemical. 7. Handling and Use of Corrosive and Contact-Hazard Chemicals. 8. Handling and Use of Reactive Chemicals: a. Shock Sensitive Chemicals. b. Safe Use of Perchloric Acid. c. Peroxide Forming Compounds. d. Classes of Peroxides. e. Water Reactive Chemicals. 9. Handling and Use of Toxic Chemicals: a. Acutely Toxic Chemicals. b. Acutely Toxic Gases. Reviewed & Revised 9-2019 Page 1 of 84 Chemical Hygiene Plan (Laboratory Safety) c. Hazards of Mercury. d. Select Carcinogens. 10. Handling and Use of Compressed Gases. 11. Lab Equipment. 12. First Aid. 13. Laboratory Chemicals of Concern. 14. Compatibility concerns in Chemical Storage. 15. Chemical Storage Plan for Laboratories: a. Color Coded Labeling Systems. b. Suggested Shelf Storage Pattern. c. National Safety Council Suggested Storage. d. Chemical Incompatibility. 16. General Laboratory Hazards and Safety Equipment. 17. Safety Guidelines for Pregnant Women in the Laboratory. a. Reproductive Hazards. 18. 74 ways to reduce hazardous waste in the laboratory. 19. Laboratory Safety Rules. XI. Laboratory Self-Survey Form XII. Laboratory Fume Hoods XIII. Laboratory Close-out Procedures and Checklist XIV. -

Non-Classical Polyinterhalides of Chlorine Monofluoride: Experimental and Theoretical Cite This: Chem
ChemComm View Article Online COMMUNICATION View Journal | View Issue Non-classical polyinterhalides of chlorine monofluoride: experimental and theoretical Cite this: Chem. Commun., 2021, À 57, 4843 characterization of [F(ClF)3] † Received 26th February 2021, Accepted 7th April 2021 Patrick Pro¨hm, Nico Schwarze, Carsten Mu¨ller, Simon Steinhauer, Helmut Beckers, Susanne M. Rupf and Sebastian Riedel * DOI: 10.1039/d1cc01088c rsc.li/chemcomm We present the synthesis and characterization of the first non- the non-classical interhalides. It was synthesized via the expo- classical Cl(I) polyinterhalide [NMe4][F(ClF)3] as well as the homo- sure of tetramethylammonium chloride to an excess of chlorine logous polychloride [NPr3Me][Cl7]. Both salts were obtained from monofluoride in dichlorofluoromethane at low temperatures Creative Commons Attribution 3.0 Unported Licence. the reaction of the corresponding ammonium chlorides with ClF or (eqn (1)). Initially, ClF likely oxidizes the chloride anion to yield Cl2, respectively. Quantum-chemical investigations predict an elemental chlorine and a fluoride anion, which is eventually À unexpected planar structure for the [F(ClF)3] anion. coordinated by three ClF molecules. We were able to grow single crystals suitable for X-ray Fluoridopolyhalogen chemistry is experiencing a renaissance. diffraction at À80 1C. [NMe4][F(ClF)3] crystallized in the orthor- In the last few decades, several binary fluoridopolyhalogenates hombic space group Pna21, as shown in Fig. 1. The anion were structurally described. They can be divided into two consists of a central fluoride anion F1 coordinated by three conceptually different groups, classical and non-classical ClF molecules in a pyramidal shape. -

Resistance of Nickel-Containing Alloys in Hydrofluoric Acid, Hydrogen Fluoride and Fluorine (Ceb-5)
CORROSION- RESISTANCE OF NICKEL-CONTAINING ALLOYS IN HYDROFLUORIC ACID, HYDROGEN FLUORIDE AND FLUORINE (CEB-5) A PRACTICAL GUIDE TO THE USE OF NICKEL-CONTAINING ALLOYS NO 443 Distributed by Produced by NICKEL INCO INSTITUTE CORROSION-RESISTANCE OF NICKEL-CONTAINING ALLOYS IN HYDROFLUORIC ACID, HYDROGEN FLUORIDE AND FLUORINE (CEB-5) A PRACTICAL GUIDE TO THE USE OF NICKEL-CONTAINING ALLOYS NO 443 Originally, this handbook was published in 1976 by INCO, The International Nickel Company, Inc. Today this company is part of Vale S.A. The Nickel Institute republished the handbook in 2020. Despite the age of this publication the information herein is considered to be generally valid. Material presented in the handbook has been prepared for the general in-formation of the reader and should not be used or relied on for specific appli-cations without first securing competent advice. The Nickel Institute, the American Iron and Steel Institute, their members, staff and consultants do not represent or warrant its suitability for any general or specific use and assume no liability or responsibility of any kind in connec-tion with the information herein. Nickel Institute [email protected] www.nickelinstitute.org Table of Contents Page Introduction ............................................................................................................................................ 3 Corrosion by Hydrofluoric Acid .......................................................................................................... 3 Nickel-Copper