An EAPCI Expert Consensus Document on Ischaemia with Non
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

I MIEI 40 ANNI Di Progettazione Alla Fiat I Miei 40 Anni Di Progettazione Alla Fiat DANTE GIACOSA
DANTE GIACOSA I MIEI 40 ANNI di progettazione alla Fiat I miei 40 anni di progettazione alla Fiat DANTE GIACOSA I MIEI 40 ANNI di progettazione alla Fiat Editing e apparati a cura di: Angelo Tito Anselmi Progettazione grafica e impaginazione: Fregi e Majuscole, Torino Due precedenti edizioni di questo volume, I miei 40 anni di progettazione alla Fiat e Progetti alla Fiat prima del computer, sono state pubblicate da Automobilia rispettivamente nel 1979 e nel 1988. Per volere della signora Mariella Zanon di Valgiurata, figlia di Dante Giacosa, questa pubblicazione ricalca fedelmente la prima edizione del 1979, anche per quanto riguarda le biografie dei protagonisti di questa storia (in cui l’unico aggiornamento è quello fornito tra parentesi quadre con la data della scomparsa laddove avve- nuta dopo il 1979). © Mariella Giacosa Zanon di Valgiurata, 1979 Ristampato nell’anno 2014 a cura di Fiat Group Marketing & Corporate Communication S.p.A. Logo di prima copertina: courtesy di Fiat Group Marketing & Corporate Communication S.p.A. … ”Noi siamo ciò di cui ci inebriamo” dice Jerry Rubin in Do it! “In ogni caso nulla ci fa più felici che parlare di noi stessi, in bene o in male. La nostra esperienza, la nostra memoria è divenuta fonte di estasi. Ed eccomi qua, io pure” Saul Bellow, Gerusalemme andata e ritorno Desidero esprimere la mia gratitudine alle persone che mi hanno incoraggiato a scrivere questo libro della mia vita di lavoro e a quelle che con il loro aiuto ne hanno reso possibile la pubblicazione. Per la sua previdente iniziativa di prender nota di incontri e fatti significativi e conservare documenti, Wanda Vigliano Mundula che mi fu vicina come segretaria dal 1946 al 1975. -
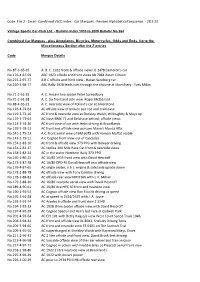
Combined VSCC Index - Car Marques - Revised Alphabetical Sequence
Code: File 2 - Excel - Combined VSCC Index - Car Marques - Revised Alphabetical Sequence. - 28.5.20. Vintage Sports Car-Club Ltd. - Bulletin Index 1935 to 2009 Bulletin No.264 Combined Car Marques - plus Aeroplanes, Bicycles, Motorcycles, Odds and Ends. Go to the Miscellaneous Section after the Z entries Code Marque Details No.87-3-65-35 A. B. C. 1922 front & offside views JS 1478 Cameron's car No.176-4-87-69 ABC 1923 offside and front views KB 7084 Aston Clinton No.215-2-97-77 A B C offside and front view - Hakan Sandberg car No.220-3-98-77 ABC Rally 1928 leads cars through the chicane at Montlhery - Yves Millot No.71-2-61-35 A. C. Anzani two seater Peter Spreadbury No.71-2-61-38 A. C. Six front and side view Roger McDonald No.88-4-65-21 A. C. nearside view of Pollard's car at Silverstone No.116-4-72-64 AC offside view of broken con rod and crankcase No.119-3-73-16 AC front & nearside view at Shelsley Walsh, Willoughby & Mays up No.119-3-73-64 AC Nash BMX 72 and Delahaye behind, offside views No.123-3-74-26 AC front view of car with Jenks driving at Brooklands No.139-3-78-13 AC front and offside view pursues Mann's Monza Alfa No.141-1-79-13 A.C. front aerial view of BM 6678 with Hamish Moffat visible No.143-3-79-11 A.C Cognac front view out of Cascades No.154-2-82-10 AC front & offside view 373 PPG with Bowyer driving No.154-2-82-27 AC replica 200 Mile Race Car front & nearside views No.158-2-83-24 AC in the water Northern Rally 373 PPO No.169-1-86-25 AC 16/80 1935 front view with David Hescroff No.173-1-87-78 AC 16/80 DPD 40 David Hescroff rear offside view No.176-4-87-65 AC single seater, o.h.c. -

I Camion Italiani Dalle Origini Agli Anni Ottanta
I camion italiani dalle origini agli anni Ottanta AISA Associazione Italiana per la Storia dell’Automobile MONOGRAFIA AISA 124 I I camion italiani dalle origini agli anni Ottanta AISA - Associazione Italiana per la Storia dell’Automobile Brescia, Fondazione Negri, 19 ottobre 2019 3 Prefazione Lorenzo Boscarelli 4 I camion italiani Massimo Condolo 15 Nasce l’autocarro italiano Antonio Amadelli Didascalia MONOGRAFIA AISA 124 1 Prefazione Lorenzo Boscarelli l camion è nato pochi anni dopo l’automobile e se di rado frutto di progetti originali, ma fruitori in buo- Ine è ben presto differenziato, per soluzioni costrut- na parte di componenti – innanzitutto il motore – di tive, dimensioni e quant’altro. Potremmo chiederci origine camionistica. Un comparto particolare sono perché si ebbe quel sia pur breve “ritardo”; un motivo le macchine movimento terra, per l’iniziale sviluppo con ogni probabilità fu che per i pionieri dell’auto- dei quali tra i paesi europei un ruolo importante ha mobile era molto più attraente fornire un mezzo di avuto l’Italia. trasporto a persone, suscitando curiosità e di diverti- La varietà degli utilizzi dei camion, da veicolo com- mento, piuttosto che offrire uno strumento per il tra- patto per consegne cittadine o di prossimità a mezzo sporto di cose. Un secondo motivo fu senza dubbio la per trasporti di grande portata, da veicolo stradale a limitatissima potenza che avevano i primi motori per mezzo da cantiere, da antincendio a cisterna aeropor- automobili, a fine Ottocento, a stento in grado di por- tuale, e chi più ne ha più ne metta, ha prodotto una tare due o quattro passeggeri, di certo non un carico varietà di soluzioni tecniche e progettuali senza pari, significativo. -

EMILIO PUGNO” CGIL Regionale Del Piemonte Camera Del Lavoro Di Torino FIOM CGIL Del Piemonte
1 ASSOCIAZIONE “EMILIO PUGNO” CGIL Regionale del Piemonte Camera del Lavoro di Torino FIOM CGIL del Piemonte Il racconto della memoria Storia della SCA a cura di Ruggero Pugno Dopo la fase decisiva di crescita degli anni ’60, il Sindacato si rinnova nei Consigli. Ma nei Consigli stessi, e, a maggior ragione, nelle strutture esterne ai luoghi di lavoro, la CGIL, e il Sindacato nella sua fase unitaria, rimane strutturalmente ambiguo. A Torino, un nucleo dirigente fondamentale attraversa queste contraddizioni mantenendo a lungo un equilibrio organizzativo e politico di forte carattere progressivo …… il cui fondamento è il potere contrattuale sui luoghi di lavoro ed una estensione dell’intervento del movimento sindacale sui temi delle riforme sociali e della programmazione economica…… su un indirizzo strategico nel quale lo sviluppo del potere contrattuale si associ, si integri con la politica delle riforme, il cui successo è legato precisamente a questa associazione e integrazione Sergio Garavini 2 Questo libro è dedicato: all’amico Michele Giannatempo, all’amico Valentino Formaggi, all’amico Franco Isnardi, ad Alessandro Tarzariol, al Signor Oddone e alla Signora Oddone Un ringraziamento a quelli che hanno contribuito e creduto alla sua realizzazione ed uno in particolare all’amico e storico Donato Antoniello 3 PREFAZIONE Il lavoro del disegnatore tecnico di carrozzeria è un’attività che nasce e si costruisce con una buona dose di perseveranza e di volontà. Deve piacere; poiché è molto selettivo ti può far stare al palo o diventare un progettista. Non esiste una laurea per il progettista di carrozzeria, esiste quella da ingegnere di meccanica, motorista, aeronautica, architettura, per cui, in carrozzeria, ti devi creare il mestiere e perfezionarlo sempre più da solo. -

The Charm of Heritage Looks to the Future
THE CHARM OF HERITAGE LOOKS TO THE FUTURE The first “real” Ferrari and the first production Lamborghini Touring Superleggera; the jewels of Lamborghini from the Miura to the Super Trofeo; the first vehicle ever built in Italy; the joy of experiencing the 1950s of the Nicolis Museum; the pink Jaguar that took over the first page of Car Life and the cutting edge models that marked turning points in the history of cars. Just a few days from the inauguration on 20 October, Auto e Moto d’Epoca in Padua reveals the marvels of the past and the common thread linking heritage to the present day. Link Foto Gallery From 20 to 23 October 2016, 4500 cars, 500 motorbikes and 1600 exhibitors will be meeting in Padua for the biggest Classic market in Europe. At Auto e Moto d’Epoca the charm of heritage is united with previews of contemporary cars based on passion that unites past and future and the continuous innovation that keeps them united. So, the show, which has become the most important in Italy - will be opening with 16 international brands, the cars that have made history and those that will do so in the future. The national previews and new products from Alfa Romeo, Abarth, Audi, Citroën, DS, Maserati, Land Rover, Jaguar, Mercedes, Pagani, Porsche, Tesla, Volkswagen and Volvo will be alongside vintage models that have marked the evolution and created the legend of each car manufacturer. Among the top pavilions are the three souls of Lamborghini’s heritage, united in a large single stand with the Lamborghini Museum, the “Polo Storico”, the Lamborghini Club Italia and an exiting panorama from the Miura to the Super Trofeo of the jewels from Sant’Agata. -

Storia. Piemonte Nicola Crepax
Storia. Piemonte Nicola Crepax Giugno 2005 Testo per Storiaindustria.it 1 Ad esclusivo uso didattico. Gli altri diritti riservati. Storia. Piemonte 1. Prima dell’industrializzazione La Fiat viene fondata nel 1899, la Olivetti nel 1908: durante la belle époque l’economia piemontese si trasforma con l’irrompere di un sistema di grandi imprese impegnate in produzioni di nuovo tipo, come la moderna industria meccanica e quella elettrica. Anche le industrie di prodotti tradizionali come le stoffe di cotone e di lana o quelle alimentari mutano il proprio assetto diventando più grandi e complesse. Le città, intanto, cambiano volto: fabbriche e uffici; case operaie e tramway; biciclette e illuminazione elettrica. Questo sviluppo, che nell’arco di pochi anni pone l’economia piemontese alla testa dell’industrializzazione del paese, è l’esito di due diversi stimoli: da un lato, la nascita delle nuove imprese rappresenta una positiva reazione all’evoluzione economica e sociale in atto, nello stesso periodo, oltre le Alpi; dall’altro, si presenta come il frutto di un lunghissimo, secolare processo di modernizzazione delle strutture produttive regionali. Questo processo di lungo periodo si articola in due sequenze principali: l’ampia fase, tra il sedicesimo e il diciottesimo secolo, in cui anche il Piemonte, pur non del tutto isolato, sconta il progressivo allontanamento della penisola dalle grandi correnti europee di sviluppo economico e sociale; una seconda fase ottocentesca, quando nel Regno di Sardegna, soprattutto durante il decennio cavouriano, e, in seguito, durante i primi anni dello stato unitario, iniziano a ripercuotersi le dinamiche innescate dalla rivoluzione industriale europea e si pongono le basi per l’industrializzazione dell’economia regionale; è l’avvio di un percorso accidentato e contraddittorio, che vede tra l’altro Torino perdere definitivamente il ruolo di capitale. -

Hierarchical Bayesian Myocardial Perfusion Quantification
Hierarchical Bayesian myocardial perfusion quantification Citation for published version (APA): Scannell, C. M., Chiribiri, A., Villa, A. D. M., Breeuwer, M., & Lee, J. (2020). Hierarchical Bayesian myocardial perfusion quantification. Medical Image Analysis, 60, [101611]. https://doi.org/10.1016/j.media.2019.101611 DOI: 10.1016/j.media.2019.101611 Document status and date: Published: 01/02/2020 Document Version: Publisher’s PDF, also known as Version of Record (includes final page, issue and volume numbers) Please check the document version of this publication: • A submitted manuscript is the version of the article upon submission and before peer-review. There can be important differences between the submitted version and the official published version of record. People interested in the research are advised to contact the author for the final version of the publication, or visit the DOI to the publisher's website. • The final author version and the galley proof are versions of the publication after peer review. • The final published version features the final layout of the paper including the volume, issue and page numbers. Link to publication General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal. -

Owners Manual Cizeta Automobili
Owners Manual Cizeta Automobili CIZETA AUTOMOBILI is a trademark of Andrea Tosetti. Filed in November 1 (2014), the CIZETA AUTOMOBILI covers Cars, Electric cars, Motor car seats, Racing cars, Racing motor cars. Current Trademark Owners. Party Name Andrea. There is only one car which makes Cizeta company known with car fans: the V16T. The car had a five-speed manual transmission, a V16 engine which was to get a Cizeta V16T nowadays can make an order with Cizeta Automobili USA in It was developed against the owner's will who did not like vehicles derived. working at Cizeta Automobili Usa. Join LinkedIn today for free. See who you know at Cizeta Automobili Usa, leverage your professional network, and get hired. Donagrandi Nicola is on Facebook. Join Facebook to connect with Donagrandi Nicola and others you may know. Facebook gives people the power to share. Or, contact the owner Andrea Tosetti of the CIZETA AUTOMOBILI trademark by line borders (Geometric figures and solids - Coding and searching guide) see. Alfa Romeo Automobili · Classic and modern Maserati, Ferrari and other Uploaded by user Italian Sports, Cizeta V16T, Sports Cars, Cars Built, Cars Interiors, Cars #luxury sports cars/ how-to-be-health-guide-9857.blogspot.com. Owners Manual Cizeta Automobili Read/Download Performance Car Guide “Lamborghini owners and enthusiasts in the UAE can now access clothing and accessories that reflect the The First Exclusive Collezione Automobili Lamborghini Fashion Outlet (5) Campagna, Caparo, Carbontech, Carver, Caterham, Chevrolet, Chrysler, Cisitalia, Citroen, Cizeta, Daihatsu. World Champions Mike Hawthorn and Juan Manuel Fangio both using them personally. anything like enough, Cizeta's outrageous V16T of the 1990s might appeal. -

Working Paper Series
(Italy) Martiis” de Torino Working Paper Series 10153 “Cognetti 09/19 100/A, Siena Statistics IL COORDINAMENTO NELLA MEDIAZIONE CIVILE E Dora and COMMERCIALE L EMERGENZA DI NORME THE BIRTH AND: ’ DEVELOPMENT OF THE ITALIANHAYEKIANE AUTOMOTIVE E IL PERCORSO INDUSTRY “PROTETTO” (1894 VERSO‐2015) Lungo AND THEL’ORDINE TURIN SOCIALE CAR CLUSTER Economics Einaudi, of Luigi ALDOANGELA ENRIETTI, AMBROSINO ALDO GEUNA, CONSUELO R. NAVA and PIER PAOLO PATRUCCO Department Campus www.est.unito.it The Department of Economics and Statistics “Cognetti de Martiis” publishes research papers authored by members and guests of the Department and of its research centers. ISSN: 2039 ‐4004 LEI&BRICK Working Paper 01/2019 www.brick.carloalberto.org The birth and development of the Italian automotive industry (1894-2015) and the Turin car cluster Aldo Enrietti Department of Economics and Statisitcs Cognetti de Martiis & ISMEL, Polo del Novecento Aldo Geuna Department of Economics and Statisitcs Cognetti de Martiis & BRICK, Collegio Carlo Alberto Consuelo R. Nava Department of Economics and Political Sciences Pier Paolo Patrucco Department of Economics and Statisitcs Cognetti de Martiis & BRICK, Collegio Carlo Alberto March 2019 Acknowledgments The original idea for this paper was made more concrete as a result of discussions between Aldo Geuna and Dan Breznitz during the latter’s period as a visiting fellow at the Collegio Carlo Alberto. The authors also acknowledge useful comments and suggestion from Paolo Bianchini, Carlo Bottai, Brendon Markey- Towler and Stefano Musso and participants in the: 2016 seminar at the Monk School of Global Affairs, Toronto University, the 2016 Schumpeter Conference, the 2016 Collegio Carlo Alberto Workshop, the DRUID 2017 and EARIE 2017 conferences and the 2018 Geoinno Conference. -

EN3. Museo Dell'automobile Tra Storia E Innovazione
MUSEO NAZIONALE DELL’AUTOMOBILE DI TORINO: BETWEEN HISTORY AND INNOVATION 30 new areas to tell the story of a symbol: from a means of transport to a cult object The story of automobiles is not only the story of a means of transport, but the events and the chronicles of nations are also narrated through it; the great events of Europe in the XX century and above all the transformations of society and of culture . What would Futurism be without its symbol of progress and modernity? The manifesto of movement states that “A roaring car that seems to ride on grapeshot is more beautiful than the Victory of Samothrace”. How can we fail to consider the increase of the diffusion of the internal combustion engine, which took place during the First World War? And can we ignore the Volkswagen van which became the universal symbol of freedom and unconventionality of “hippies” in the 60s and 70s? The lasting connection between the story of automobiles and the story of our society and culture is represented by Torino’s Museo Nazionale dell’Automobile through 30 sections that cover over 9,000 square metres. These are broken down into a room repartition that precisely define different topics and specific historical periods. This provides visitors with a clear narrative itinerary, expressed by titles and settings, from the origins to nowadays. The journey starts on the second floor by the GENESI (Genesis) section; a homage to all the forerunners who, during the last five centuries, have been looking for a way to move and carry things and people without the help of animals. -

Doing Something Interesting
March-April1995 Issue Number 155 several sizes. While the thrust of this enterprise is not to serve the historical community per se (Robertson anticipates that "a brisk demand will develop with the photographs being used as theme decor in such places as restaurants, offices, or GM dealerships."), it may be able to provide images previously unknown (or believed lost) for researchers and journalists. For further information on the GM Media Archives, contact John Robertson at General Motors Corporation, Argonaut B. Building, 8th Floor, 465 West Milwau BRIGHAM RECUPERATES; kee Avenue, Detroit, MI 48282 USA. APPRECIATES HEARING FROM MEMBERS SAH's ITALIAN COUNTERPART SAH co-founder Dick Brigham recently returned home after Several years ago, SAH established a publications exchange hospitalization for a slight fracture and pneumonia. During his with the Associazone ltaliana per Ia Storia deii'Automobile (AISA), convalescence he "will enjoy talking again with members ofSAH," which recently provided a list of its 1994 activities. according to his wife Grace, also a founding and honorary member AISA's home is the Biscaretti Automobile Museum in Torino, of the Society. The Brighams were jointly recognized as Friends of but its meetings are conducted at various places in Northern Italy. Automotive History in 1985. A program on the Lancia Aurelia and its V6 engine was held at the As Dick lost his sight following a stroke in 1989, it is best to National Museum of Science and Technology in Milano, while a reach him by telephone at (404) 422-9115. round table discussion of Abarth was followed by a visit to the Caproni Aeronautical Museum in Trento. -

Forme E Creatività Dell'automobile Cento Anni Di Carrozzeria 1911-2011
Forme e creatività dell’automobile cento anni di carrozzeria 1911-2011 AISA - Associazione Italiana per la Storia dell’Automobile AISA • Associazione Italiana per la Storia dell’Automobile MONOGRAFIA AISA 94 C.so di Porta Vigentina, 32 - 20122 Milano - www.aisastoryauto.it Forme e creatività dell’automobile cento anni di carrozzeria 1911-2011 AISA - Associazione Italiana per la Storia dell’Automobile in collaborazione con Museo Nazionale dell’Automobile di Torino Torino, 29 ottobre 2011 3 Introduzione Lorenzo Boscarelli 4 I carrozzieri e la Fiat: cento anni di collaborazione Alessandro Sannia 18 I miei anni alla Zagato Ercole Spada 22 Prospettive per i carrozzieri di domani Leonardo Fioravanti In copertina: la concept-car “Sguanà” realizzata da Bertone nel 2006 su base Fiat Grande Punto ed una Fiat 525 allestita con carrozzeria D’Orsay de Ville da Viotti nel 1928. In IV di copertina: una panoramica su un secolo di vetture Fiat “vestite” dai carrozzieri italiani. MONOGRAFIA AISA 94 1 Prefazione Lorenzo Boscarelli n secolo è un lungo periodo per un prodotto dell’in- laborazione con i carrozzieri un contributo rilevante all’af- Udustria, come l’automobile, tanto più se nel suo corso fermazione dei loro prodotti. Possiamo ben dire che questo le evoluzioni della tecnologia, dell’economia, della società risultato è stata la conseguenza di un virtuoso “triangolo”, hanno inciso così profondamente sull’oggetto e sul suo i cui vertici sono rappresentati dall’azienda committente, ambiente da rendere arduo il paragone tra i due estremi dall’impresa di carrozzeria, dallo stilista (oggi detto desi- temporali. gner), con questi ultimi due attori che hanno saputo evol- È quindi ancor più ammirevole e – per noi italiani – occa- vere nel tempo, assumendo connotazioni e ruoli consoni sione di orgoglio, che si possa celebrare il centesimo anni- alle mutate condizioni tecniche, organizzative, di mercato.