Commonly Administered Vaccines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety of Immunization During Pregnancy a Review of the Evidence
Safety of Immunization during Pregnancy A review of the evidence Global Advisory Committee on Vaccine Safety © World Health Organization 2014 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications –whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO website (www.who.int/about/licensing/copyright_form/en/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. -

Pneumococcal Vaccine
Vaccines for Primary Care Pneumococcal, Shingles, Pertussis Devang Patel, M.D. Assistant Professor Chief of Service, MICU ID Service University of Maryland School of Medicine Pneumococcal Vaccine Pneumococcal Disease • 2nd most common cause of vaccine preventable death in the US • Major Syndromes – Pneumonia – Bacteremia – Meningitis Active Bacterial Core Surveillance (ABCs) Report Emerging Infections Program Network Streptococcus pneumoniae, 2010 (ORIG) Vaccine Target • Polysaccharide capsule allows bacteria to resist phagocytosis • Antibodies to capsule facilitate phagocytosis • >90 different pneumococcal capsular serotypes • Vaccines contain most common serotypes causing disease Pneumococcal Vaccines Pneumococcal Vaccines • Pneumococcal polysaccharide vaccine (PPSV23; Pneumovax) – Contains capsular polysaccharides – 23 most commonly infecting serotypes • Cause 60% of all pneumococcal infections in adults – Not recommended for children <2 due to poor immunogenicity of polysaccharides Pneumococcal Vaccines • Pneumococcal conjugate vaccine (PCV13, Prevnar) – Polysaccharides linked to nontoxic protein • higher antigenicity – Stimulates mucosal antibody • Eliminates nasal carriage in young children • Herd effect in adults – Reduction in PCV7 serotype disease >90% Prevnar 13 • 2000 - PCV7 approved for infants toddlers • 2010 - PCV13 recommended for infants and toddlers • 2012 – ACIP recommended PCV13 for high- risk adults • 2014 – recommended for adults >65 • 2018 – ACIP will revisit PCV13 use in adults – Childhood vaccines may eliminate -

Summary Guide to Tetanus Prophylaxis in Routine Wound Management
Summary Guide to Tetanus Prophylaxis in Routine Wound Management ASSESS WOUND A clean, minor wound All other wounds (contaminated with dirt, feces, saliva, soil; puncture wounds; avulsions; wounds resulting from flying or crushing objects, animal bites, burns, frostbite) Has patient completed a primary Has patient completed a primary tetanus diphtheria series?1, 7 tetanus diphtheria series?1, 7 No/Unknown Yes No/Unknown Yes Administer vaccine today.2,3,4 Was the most recent Administer vaccine and Was the most Instruct patient to complete dose within the past tetanus immune gobulin recent dose within series per age-appropriate 10 years? (TIG) now.2,4,5,6,7 the past 5 years?7 vaccine schedule. No Yes No Yes Vaccine not needed today. Vaccine not needed today. Administer vaccine today.2,4 2,4 Patient should receive next Administer vaccine today. Patient should receive next Patient should receive next dose Patient should receive next dose dose at 10-year interval after dose at 10-year interval after per age-appropriate schedule. per age-appropriate schedule. last dose. last dose. 1 A primary series consists of a minimum of 3 doses of tetanus- and diphtheria- 4 Tdap* is preferred for persons 11 through 64 years of age if using Adacel* or 10 years of containing vaccine (DTaP/DTP/Tdap/DT/Td). age and older if using Boostrix* who have never received Tdap. Td is preferred to tetanus 2 Age-appropriate vaccine: toxoid (TT) for persons 7 through 9 years, 65 years and older, or who have received a • DTaP for infants and children 6 weeks up to 7 years of age (or DT pediatric if Tdap previously. -

YF-VAX® Rx Only AHFS Category
YF-VAX® Rx Only Laboratory Personnel AHFS Category: 80:12 Laboratory personnel who handle virulent yellow fever virus or concentrated preparations Rx only of the yellow fever vaccine virus strains may be at risk of exposure by direct or indirect Yellow Fever Vaccine contact or by aerosols. (14) DESCRIPTION CONTRAINDICATIONS ® Hypersensitivity YF-VAX , Yellow Fever Vaccine, for subcutaneous use, is prepared by culturing the YF-VAX is contraindicated in anyone with a history of acute hypersensitivity reaction to any 17D-204 strain of yellow fever virus in living avian leukosis virus-free (ALV-free) chicken component of the vaccine. (See DESCRIPTION section.) Because the yellow fever virus embryos. The vaccine contains sorbitol and gelatin as a stabilizer, is lyophilized, and is used in the production of this vaccine is propagated in chicken embryos, do not administer hermetically sealed under nitrogen. No preservative is added. Each vial of vaccine is YF-VAX to anyone with a history of acute hypersensitivity to eggs or egg products due to supplied with a separate vial of sterile diluent, which contains Sodium Chloride Injection a risk of anaphylaxis. Less severe or localized manifestations of allergy to eggs or to USP – without a preservative. YF-VAX is formulated to contain not less than 4.74 log10 feathers are not contraindications to vaccine administration and do not usually warrant plaque forming units (PFU) per 0.5 mL dose throughout the life of the product. Before vaccine skin testing. (See PRECAUTIONS section, Testing for Hypersensitivity Re- reconstitution, YF-VAX is a pinkish color. After reconstitution, YF-VAX is a slight pink-brown actions subsection.) Generally, persons who are able to eat eggs or egg products may suspension. -

Vaccines for Preteens
| DISEASES and the VACCINES THAT PREVENT THEM | INFORMATION FOR PARENTS Vaccines for Preteens: What Parents Should Know Last updated JANUARY 2017 Why does my child need vaccines now? to get vaccinated. The best time to get the flu vaccine is as soon as it’s available in your community, ideally by October. Vaccines aren’t just for babies. Some of the vaccines that While it’s best to be vaccinated before flu begins causing babies get can wear off as kids get older. And as kids grow up illness in your community, flu vaccination can be beneficial as they may come in contact with different diseases than when long as flu viruses are circulating, even in January or later. they were babies. There are vaccines that can help protect your preteen or teen from these other illnesses. When should my child be vaccinated? What vaccines does my child need? A good time to get these vaccines is during a yearly health Tdap Vaccine checkup. Your preteen or teen can also get these vaccines at This vaccine helps protect against three serious diseases: a physical exam required for sports, school, or camp. It’s a tetanus, diphtheria, and pertussis (whooping cough). good idea to ask the doctor or nurse every year if there are any Preteens should get Tdap at age 11 or 12. If your teen didn’t vaccines that your child may need. get a Tdap shot as a preteen, ask their doctor or nurse about getting the shot now. What else should I know about these vaccines? These vaccines have all been studied very carefully and are Meningococcal Vaccine safe. -

Vaccine Hesitancy
Vaccine Hesitancy Dr Brenda Corcoran National Immunisation Office Presentation Outline An understanding of the following principles: • Overview of immunity • Different types of vaccines and vaccine contents • Vaccine failures • Time intervals between vaccine doses • Vaccine overload • Adverse reactions • Herd immunity Immunity Immunity • The ability of the human body to protect itself from infectious disease The immune system • Cells with a protective function in the – bone marrow – thymus – lymphatic system of ducts and nodes – spleen –blood Types of immunity Source: http://en.wikipedia.org/wiki/Immunological_memory Natural (innate) immunity Non-specific mechanisms – Physical barriers • skin and mucous membranes – Chemical barriers • gastric and digestive enzymes – Cellular and protein secretions • phagocytes, macrophages, complement system ** No “memory” of protection exists afterwards ** Passive immunity – adaptive mechanisms Natural • maternal transfer of antibodies to infant via placenta Artificial • administration of pre- formed substance to provide immediate but short-term protection (antitoxin, antibodies) Protection is temporary and wanes with time (usually few months) Active immunity – adaptive mechanisms Natural • following contact with organism Artificial • administration of agent to stimulate immune response (immunisation) Acquired through contact with an micro-organism Protection produced by individual’s own immune system Protection often life-long but may need boosting How vaccines work • Induce active immunity – Immunity and immunologic memory similar to natural infection but without risk of disease • Immunological memory allows – Rapid recognition and response to pathogen – Prevent or modify effect of disease Live attenuated vaccines Weakened viruses /bacteria – Achieved by growing numerous generations in laboratory – Produces long lasting immune response after one or two doses – Stimulates immune system to react as it does to natural infection – Can cause mild form of the disease (e.g. -

Ensuring the World's Poorest Children Benefit from Lifesaving Vaccines
WHAT WE’RE LEARNING Ensuring the World’s Poorest Children Benefit from Lifesaving Vaccines “Supporting children’s immunization is undoubtedly the best investment we’ve ever made.” —Bill and Melinda Gates Program Area pertussis) vaccine. When GAVI was The Challenge launched, 71 percent of the world’s Global Health Most people in rich nations do not children were receiving DTP vaccine. realize how difficult it is simply to By 2004, the rate had increased to survive early childhood in many parts Our Goal 78 percent. of the world. Every year, more than Prevent needless deaths in the world’s GAVI has concentrated the greatest 10 million children are buried before poorest countries by increasing access percentage of its resources on speeding they reach their fifth birthday. Of these, to basic vaccines and speeding the the introduction of newer, more about 1.4 million die from diseases that introduction of new vaccines. expensive vaccines, including those for existing vaccines can prevent—including hepatitis B, Haemophilus influenzae B measles, pertussis, and tetanus. Another Our Progress in Brief (Hib), and yellow fever. The vaccine 1.1 million die from diseases for which The Global Alliance for Vaccines for hepatitis B, for example, had been we will soon have vaccines. Because and Immunization (GAVI), which widely used in rich countries for childhood death is so frequent, parents the foundation helped to launch in 20 years but was severely underutilized in some countries in Africa and Asia 1999, has made significant progress. in the developing world because of a don’t even name their babies until they Children’s immunization is no longer a lack of political will and the vaccine’s reach several months of age. -

Association Between Thimerosal-Containing Vaccine and Autism
BRIEF REPORT Association Between Thimerosal-Containing Vaccine and Autism Anders Hviid, MSc Context Mercuric compounds are nephrotoxic and neurotoxic at high doses. Thi- Michael Stellfeld, MD merosal, a preservative used widely in vaccine formulations, contains ethylmercury. Thus it has been suggested that childhood vaccination with thimerosal-containing vac- Jan Wohlfahrt, MSc cine could be causally related to neurodevelopmental disorders such as autism. Mads Melbye, MD, PhD Objective To determine whether vaccination with a thimerosal-containing vaccine is associated with development of autism. IGH DOSES OF MERCURIC COM- Design, Setting, and Participants Population-based cohort study of all children pounds are nephrotoxic and born in Denmark from January 1, 1990, until December 31, 1996 (N=467450) com- neurotoxic.1 Thimerosal, an paring children vaccinated with a thimerosal-containing vaccine with children vacci- organic compound that con- nated with a thimerosal-free formulation of the same vaccine. Htains ethylmercury, has been widely used Main Outcome Measures Rate ratio (RR) for autism and other autistic-spectrum since the 1930s as a preservative in cer- disorders, including trend with dose of ethylmercury. tain vaccines. In the 1990s, an increas- Results During 2986654 person-years, we identified 440 autism cases and 787 cases ing number of different vaccines con- of other autistic-spectrum disorders. The risk of autism and other autistic-spectrum taining thimerosal were introduced in disorders did not differ significantly between children vaccinated with thimerosal- immunization schedules around the containing vaccine and children vaccinated with thimerosal-free vaccine (RR, 0.85 [95% world, and thus the average cumula- confidence interval {CI}, 0.60-1.20] for autism; RR, 1.12 [95% CI, 0.88-1.43] for other tive exposure to thimerosal in infants has autistic-spectrum disorders). -

Criteria for Reviewing Pneumococcal Conjugate Vaccine
Draft—3/11/10 Oregon School/Facility Immunization Advisory Committee: Review of Pneumococcal Conjugate Vaccine Against Twelve Criteria for Children’s Facility Immunization Requirements Oregon Department of Human Services Public Health Division Office of Family Health Immunization Program 800 NE Oregon Street, Suite 370 Portland, Oregon 97232 Phone: 971-673-0300 Fax: 971-673-0278 Web: www.oregon.gov/DHS/ph/imm Page 1–Review of Pneumococcal Conjugate Vaccine Against Criteria for School Law Immunization Requirements Oregon School/Facility Immunization Advisory Committee: Review of Pneumococcal Conjugate Vaccine Against Twelve Criteria for School/Facility/College Immunization Requirements Process for Reviewing Antigens for Potential Inclusion in OAR 333-050-0050, 333-050-0130 and 333-050-0140. Request for the inclusion of additional antigens or vaccines can come from the Oregon Immunization Program, IPAT (Immunization Policy Advisory Team), or from the community. Proposed changes to vaccine requirements are discussed with IPAT either in a regularly scheduled meeting or through electronic communication. IPAT will submit their comments and a request for consideration to the Oregon Immunization School Law Advisory Committee. The Oregon School/Facility Immunization Advisory Committee was established as a part of the school law immunization requirements when the original legislation was passed in 1980. This Committee is composed of immunization stakeholders from the fields of public health, school health, school administration, medicine, day care, child advocacy and consumers (parents). Through consensus, the committee determines what vaccines (antigens) should be included in Oregon school immunization requirements. Information about new vaccines and the diseases they prevent, including transmission within schools, burden of disease, cost-effectiveness, effect on schools/counties and vaccine availability is presented at a scheduled meeting for committee consideration. -

Immunity and How Vaccines Work
Immunity and how vaccines work Dr Brenda Corcoran National Immunisation Office Presentation Outline An understanding of the following principles: • Overview of immunity • Different types of vaccines and vaccine contents • Vaccine failures • Time intervals between vaccine doses • Vaccine overload • Adverse reactions • Herd immunity Immunity Immunity • The ability of the human body to protect itself from infectious disease The immune system • Cells with a protective function in the – bone marrow – thymus – lymphatic system of ducts and nodes – spleen –blood Types of immunity Source: http://en.wikipedia.org/wiki/Immunological_memory Natural (innate) immunity Non-specific mechanisms – Physical barriers • skin and mucous membranes – Chemical barriers • gastric and digestive enzymes – Cellular and protein secretions • phagocytes, macrophages, complement system ** No “memory” of protection exists afterwards ** Passive immunity – adaptive mechanisms Natural • maternal transfer of antibodies to infant via placenta Artificial • administration of pre- formed substance to provide immediate but short-term protection (antitoxin, antibodies) Protection is temporary and wanes with time (usually few months) Active immunity – adaptive mechanisms Natural • following contact with organism Artificial • administration of agent to stimulate immune response (immunisation) Acquired through contact with an micro-organism Protection produced by individual’s own immune system Protection often life-long but may need boosting How vaccines work • Induce active immunity – Immunity and immunologic memory similar to natural infection but without risk of disease • Immunological memory allows – Rapid recognition and response to pathogen – Prevent or modify effect of disease Live attenuated vaccines Weakened viruses /bacteria – Achieved by growing numerous generations in laboratory – Produces long lasting immune response after one or two doses – Stimulates immune system to react as it does to natural infection – Can cause mild form of the disease (e.g. -

2021 Medicare Vaccine Coverage Part B Vs Part D
CDPHP® Medicare Advantage Vaccine Coverage Guide Part B (Medical) vs. Part D (Pharmacy) Medicare Part B (Medical): Medicare Part D (Pharmacy): Vaccinations or inoculations Vaccinations or inoculations are included when the administration is (except influenza, pneumococcal, reasonable and necessary for the prevention of illness. and hepatitis B for members at risk) are excluded unless they are directly related to the treatment of an injury or direct exposure to a disease or condition. • Influenza Vaccine (Flu) • BCG Vaccine • Pneumococcal Vaccine • Diphtheria/Tetanus/Acellular Pertussis Vaccine (ADACEL, (Pneumovax, Prevnar 13) BOOSTRIX, DAPTACEL, INFANRIX) • Hepatitis B Vaccine • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus (Recombivax, Engerix-B) Vaccine (KINRIX, QUADRACEL) for members at moderate • Diphtheria/Tetanus Vaccine (DT, Td, TDVAX, TENIVAC) to high risk • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus Vac • Other vaccines when directly cine/ Haemophilus Influenzae Type B Conjugate Vaccine (PENTACEL) related to the treatment of an • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus injury or direct exposure to a Vaccine/Hepatitis B Vaccine (PEDIARIX) disease or condition, such as: • Haemophilus Influenzae Type B Conjugate Vaccine (ActHIB, PedvaxHIB, • Antivenom Sera Hiberix) • Diphtheria/Tetanus Vaccine • Hepatitis A Vaccine, Inactivated (VAQTA) (DT, Td, TDVAX, TENIVAC) • Hepatitis B Vaccine, Recombinant (ENGERIX-B, RECOMBIVAX HB) • Rabies Virus Vaccine for members at low risk (RabAvert, -
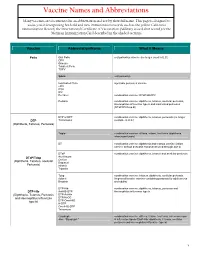
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus,