Vaccine Hesitancy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary Guide to Tetanus Prophylaxis in Routine Wound Management
Summary Guide to Tetanus Prophylaxis in Routine Wound Management ASSESS WOUND A clean, minor wound All other wounds (contaminated with dirt, feces, saliva, soil; puncture wounds; avulsions; wounds resulting from flying or crushing objects, animal bites, burns, frostbite) Has patient completed a primary Has patient completed a primary tetanus diphtheria series?1, 7 tetanus diphtheria series?1, 7 No/Unknown Yes No/Unknown Yes Administer vaccine today.2,3,4 Was the most recent Administer vaccine and Was the most Instruct patient to complete dose within the past tetanus immune gobulin recent dose within series per age-appropriate 10 years? (TIG) now.2,4,5,6,7 the past 5 years?7 vaccine schedule. No Yes No Yes Vaccine not needed today. Vaccine not needed today. Administer vaccine today.2,4 2,4 Patient should receive next Administer vaccine today. Patient should receive next Patient should receive next dose Patient should receive next dose dose at 10-year interval after dose at 10-year interval after per age-appropriate schedule. per age-appropriate schedule. last dose. last dose. 1 A primary series consists of a minimum of 3 doses of tetanus- and diphtheria- 4 Tdap* is preferred for persons 11 through 64 years of age if using Adacel* or 10 years of containing vaccine (DTaP/DTP/Tdap/DT/Td). age and older if using Boostrix* who have never received Tdap. Td is preferred to tetanus 2 Age-appropriate vaccine: toxoid (TT) for persons 7 through 9 years, 65 years and older, or who have received a • DTaP for infants and children 6 weeks up to 7 years of age (or DT pediatric if Tdap previously. -

Vaccines 101 the Very Last Day That I Was a Pediatric Resident. Um, Many
Vaccines 101 The very last day that I was a pediatric resident. Um, many years ago, a toddler walked into the emergency room and uh, and progressively got sicker and sicker. That's Dr. Katherine Edwards, a world expert in pediatric infectious disease in vaccinology. She's also a professor of pediatrics at Vanderbilt university and she's been working on vaccines for 40 years. I did a spinal tap on her and realized that she had Haemophilus influenza, typ e B meningitis, Haemophilus influenza, type B or HIB is it bacteria normally found in our nose and throat that can lead to very serious life threatening infections and no matter what I did in that day and into the night in terms of prompt antibiotics and she'd just been sick a few hours and, and fluids and all the, you know, ventilators and all the best things that modern medicine, she died, the vaccine for hip was not available until the 1990s. And until it did become available, hip disease affected approximately 25,000 children each year with things like meningitis, pneumonia, and bloodstream infections. And at that time in the hospital that I was practicing at any one time, there were generally five or six patients that had Haemophilus meningitis or invasive disease or some complication of this particular infection. And we knew that from the basic science that if you had antibody to the capsule or to the coat of the organism, that you were protected from disease. But we really didn't know how to make little kids make antibody. -

Commonly Administered Vaccines
Commonly Administered Vaccines CPT CVX Vaccine Type Brand Name Manufacturer PhilaVax Display Name Description Code Code Combination Vaccines Diptheria, tetanus toxoids and acellular pertussis vaccine, Hepati- DTaP-HepB-IPV Pediarix® GlaxoSmithKline 90723 110 DTaP-HepB-IPV tis B and poliovirus vaccine, inactivated Diptheria, tetanus toxoids and acellular pertussis vaccine and DTaP-Hib TriHIBit® Sanofi Pasteur 90721 50 DTaP-Hib (TriHIBit) Haemophilus influenzae type b conjugate vaccine Diptheria, tetanus toxoids and acellular pertussis vaccine, Hae- DTaP-Hib-IPV Pentacel® Sanofi Pasteur 90698 120 DTaP-Hib-IPV mophilus influenzae type b, and poliovirus vaccine, inactivated Diptheria, tetanus toxoids and acellular pertussis vaccine, and DTaP-IPV Kinrix® GlaxoSmithKline 90696 130 DTaP-IPV (KINRIX) poliovirus vaccine, inactivated HepA-HepB TWINRIX® GlaxoSmithKline 90636 104 HepA/B (TWINRIX) Hepatisis A and Hepatitis B vaccine, adult dosage Hepatitis B and Hemophilus influenza b vaccine, for intramuscular HepB-Hib Comvax® Merck 90748 51 Hib-Hep B (COMVAX) use Haemophilus influenza b and meningococcal sero groups C and Y MeningC/Y-Hib Menhibrix® GlaxoSmithKline 90644 148 Meningococcal-Hib vaccine, 4 dose series Diphtheria, Tetanus and Pertussis DTaP Infanrix® GlaxoSmithKline 90700 20 DTaP (INFANRIX) Diptheria, tetanus toxoids and acellular pertussis vaccine DTaP, 5 Pertussis Antigens Daptacel® Sanofi Pasteur 90700 106 DTaP (DAPTACEL) Diptheria, tetanus toxoids and acellular pertussis vaccine Tetanus toxoid, reduced diphtheria toxoid, and acellular -

The Challenge of Achieving Universal Access to Vaccines
Keynote Address: The Challenge of Achieving Universal Access to Vaccines WIPO & AMF Seth Berkley M.D, CEO 8 November 2017 www.gavi.org 1 Vaccine landscape WIPO 8 November 2017 The world before vaccines Examples of major disease outbreaks Polio Cholera New York pandemic 1916 Europe 6,000 deaths 1829-1851 >200,000 deaths Yellow fever Philadelphia Smallpox 1793 epidemic >5,000 India deaths 1974 15,000 deaths Flu pandemic 1918-1920 > 50-100 million deaths worldwide WIPO 8 November 2017 History of vaccine development Source: Delaney et.al. 2014 WIPO 8 November 2017 Cumulative number of vaccines developed licensed 50+ vaccines Lyme OspA, protein (1998) Tuberculosis (Bacille Calmette-Guérin) Pneumococcal conjugate, heptavalent* (2000) 1927 Typhus Varicella (1995) Meningococcal quadrivalent conjugates* (2005) Japanese encephalitis (Vera-cell) (2009) 1938 Cholera (recombinant Toxin B) # (1993) Cholera (WC only) (2009) Tetanus toxoid Cholera (WC-rBC) (1991) Human papillomavirus recombinant bivalent (2009) Influenza H. influenzaeconjugate* (1987) Quadrivalent influenza vaccines (2012) Typhoid Cholera Pertussis 1936 Meningococcal C and Y and 1886 1896 1926 H. influenza type B polysaccharide (1985) Haemophilusinfluenza type b (2012) Adenovirus (1980) Meningococcal B (2013) Smallpox Diphtheria Yellow Rabies, cell culture (1980) Influenza vaccine (baculovirus) (2013) 1798 toxoid Fever Typhoid conjugate vaccine (2013) Rabies Plague Meningococcal polysaccharides (1974) 1923 1935 Human papillomavirus, 9-valent (2014) 1885 Rubella, live (1969) 1897 vaccines -

Package Inserts
Individuals using assistive technology may not be able to fully access the information contained in this file. For assistance, please send an e-mail to: [email protected] and include 508 Accommodation and the title of the document in the subject line of your e-mail. HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------WARNINGS AND PRECAUTIONS------------------------ These highlights do not include all the information needed to use • Carefully consider benefits and risks before administering VAXELIS to VAXELIS safely and effectively. See full prescribing information for persons with a history of: VAXELIS. - fever ≥40.5°C (≥105°F), hypotonic-hyporesponsive episode (HHE) or VAXELISTM (Diphtheria and Tetanus Toxoids and Acellular Pertussis, persistent, inconsolable crying lasting ≥3 hours within 48 hours after a Inactivated Poliovirus, Haemophilus b Conjugate and Hepatitis B previous pertussis-containing vaccine. (5.2) Vaccine) - seizures within 3 days after a previous pertussis-containing vaccine. (5.2) Suspension for Intramuscular Injection Initial U.S. Approval: 2018 • If Guillain-Barré syndrome occurred within 6 weeks of receipt of a prior vaccine containing tetanus toxoid, the risk for Guillain-Barré syndrome ----------------------------RECENT MAJOR CHANGES ------------------------ may be increased following VAXELIS. (5.3) Dosage and Administration (2.2) 09/2020 • Apnea following intramuscular vaccination has been observed in some ----------------------------INDICATIONS AND USAGE--------------------------- infants born prematurely. The decision about when to administer an VAXELIS is a vaccine indicated for active immunization to prevent intramuscular vaccine, including VAXELIS, to an infant born prematurely diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, and invasive disease should be based on consideration of the individual infant’s medical status due to Haemophilus influenzae type b. -

Evaluation of the First Five Years of GAVI Immunization Services Support Funding
Cambridge, MA Lexington, MA Evaluation of the Hadley, MA First Five Years of Bethesda, MD Chicago, IL GAVI Immunization Services Support Funding Prepared for The GAVI Alliance Grace Chee Abt Associates Inc. Natasha Hsi Abt Associates Inc. Kenneth Carlson Abt Associates Inc. Slavea Chankova Abt Associates Inc. Patricia Taylor John Snow, Inc. September 2007 Evaluation of the First Five Years’ of GAVI Immunization Services Support Funding is a publication of Abt Associates, commissioned by the GAVI Alliance. The opinions expressed herein are those of the authors and do not necessarily reflect the views of Abt Associates, the GAVI Alliance or any of its alliance partners. September 2007 Recommended Citation Grace Chee, Natasha Hsi, Kenneth Carlson, Slavea Chankova, Patricia Taylor. September 2007. Evaluation of the First Five Years’ of GAVI Immunization Services Support Funding. Bethesda, MD: Abt Associates Inc. CONTENTS Acronyms....................................................................................................................................... v Acknowledgements...................................................................................................................... vii Executive Summary ...................................................................................................................... ix 1. Background to the Study........................................................................................................ 1 2. Approach and Methods ......................................................................................................... -

Criteria for Reviewing Pneumococcal Conjugate Vaccine
Draft—3/11/10 Oregon School/Facility Immunization Advisory Committee: Review of Pneumococcal Conjugate Vaccine Against Twelve Criteria for Children’s Facility Immunization Requirements Oregon Department of Human Services Public Health Division Office of Family Health Immunization Program 800 NE Oregon Street, Suite 370 Portland, Oregon 97232 Phone: 971-673-0300 Fax: 971-673-0278 Web: www.oregon.gov/DHS/ph/imm Page 1–Review of Pneumococcal Conjugate Vaccine Against Criteria for School Law Immunization Requirements Oregon School/Facility Immunization Advisory Committee: Review of Pneumococcal Conjugate Vaccine Against Twelve Criteria for School/Facility/College Immunization Requirements Process for Reviewing Antigens for Potential Inclusion in OAR 333-050-0050, 333-050-0130 and 333-050-0140. Request for the inclusion of additional antigens or vaccines can come from the Oregon Immunization Program, IPAT (Immunization Policy Advisory Team), or from the community. Proposed changes to vaccine requirements are discussed with IPAT either in a regularly scheduled meeting or through electronic communication. IPAT will submit their comments and a request for consideration to the Oregon Immunization School Law Advisory Committee. The Oregon School/Facility Immunization Advisory Committee was established as a part of the school law immunization requirements when the original legislation was passed in 1980. This Committee is composed of immunization stakeholders from the fields of public health, school health, school administration, medicine, day care, child advocacy and consumers (parents). Through consensus, the committee determines what vaccines (antigens) should be included in Oregon school immunization requirements. Information about new vaccines and the diseases they prevent, including transmission within schools, burden of disease, cost-effectiveness, effect on schools/counties and vaccine availability is presented at a scheduled meeting for committee consideration. -

Immunity and How Vaccines Work
Immunity and how vaccines work Dr Brenda Corcoran National Immunisation Office Presentation Outline An understanding of the following principles: • Overview of immunity • Different types of vaccines and vaccine contents • Vaccine failures • Time intervals between vaccine doses • Vaccine overload • Adverse reactions • Herd immunity Immunity Immunity • The ability of the human body to protect itself from infectious disease The immune system • Cells with a protective function in the – bone marrow – thymus – lymphatic system of ducts and nodes – spleen –blood Types of immunity Source: http://en.wikipedia.org/wiki/Immunological_memory Natural (innate) immunity Non-specific mechanisms – Physical barriers • skin and mucous membranes – Chemical barriers • gastric and digestive enzymes – Cellular and protein secretions • phagocytes, macrophages, complement system ** No “memory” of protection exists afterwards ** Passive immunity – adaptive mechanisms Natural • maternal transfer of antibodies to infant via placenta Artificial • administration of pre- formed substance to provide immediate but short-term protection (antitoxin, antibodies) Protection is temporary and wanes with time (usually few months) Active immunity – adaptive mechanisms Natural • following contact with organism Artificial • administration of agent to stimulate immune response (immunisation) Acquired through contact with an micro-organism Protection produced by individual’s own immune system Protection often life-long but may need boosting How vaccines work • Induce active immunity – Immunity and immunologic memory similar to natural infection but without risk of disease • Immunological memory allows – Rapid recognition and response to pathogen – Prevent or modify effect of disease Live attenuated vaccines Weakened viruses /bacteria – Achieved by growing numerous generations in laboratory – Produces long lasting immune response after one or two doses – Stimulates immune system to react as it does to natural infection – Can cause mild form of the disease (e.g. -

2021 Medicare Vaccine Coverage Part B Vs Part D
CDPHP® Medicare Advantage Vaccine Coverage Guide Part B (Medical) vs. Part D (Pharmacy) Medicare Part B (Medical): Medicare Part D (Pharmacy): Vaccinations or inoculations Vaccinations or inoculations are included when the administration is (except influenza, pneumococcal, reasonable and necessary for the prevention of illness. and hepatitis B for members at risk) are excluded unless they are directly related to the treatment of an injury or direct exposure to a disease or condition. • Influenza Vaccine (Flu) • BCG Vaccine • Pneumococcal Vaccine • Diphtheria/Tetanus/Acellular Pertussis Vaccine (ADACEL, (Pneumovax, Prevnar 13) BOOSTRIX, DAPTACEL, INFANRIX) • Hepatitis B Vaccine • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus (Recombivax, Engerix-B) Vaccine (KINRIX, QUADRACEL) for members at moderate • Diphtheria/Tetanus Vaccine (DT, Td, TDVAX, TENIVAC) to high risk • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus Vac • Other vaccines when directly cine/ Haemophilus Influenzae Type B Conjugate Vaccine (PENTACEL) related to the treatment of an • Diphtheria/Tetanus/Acellular Pertussis/Inactivated Poliovirus injury or direct exposure to a Vaccine/Hepatitis B Vaccine (PEDIARIX) disease or condition, such as: • Haemophilus Influenzae Type B Conjugate Vaccine (ActHIB, PedvaxHIB, • Antivenom Sera Hiberix) • Diphtheria/Tetanus Vaccine • Hepatitis A Vaccine, Inactivated (VAQTA) (DT, Td, TDVAX, TENIVAC) • Hepatitis B Vaccine, Recombinant (ENGERIX-B, RECOMBIVAX HB) • Rabies Virus Vaccine for members at low risk (RabAvert, -
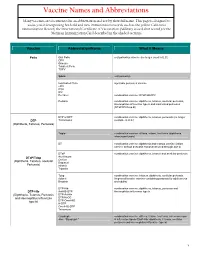
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus, -

Vaccine Adverse Event Reporting System (VAERS)
Appendix D Vaccine Adverse Event Reporting System (VAERS) VAERS is a national post-licensure vaccine safety surveillance program co-managed by the Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA). VAERS serves as an early warning system to detect possible safety issues with U.S. vaccines by collecting information about adverse events (possible side effects or health problems) that occur after vaccination. A report to VAERS does not indicate that a vaccine caused an adverse event, only that the adverse event occurred sometime after vaccination. Who can report? Anyone can submit a report to VAERS — healthcare professionals, vaccine manufacturers, and the general public. VAERS welcomes all reports, regardless of seriousness, and regardless of how likely the vaccine may have been to have caused the adverse event. What should be reported? Healthcare providers are required by law to report: y Any adverse event listed by the vaccine manufacturer as a contraindication to subsequent doses of the vaccine y Any adverse event listed in the Reportable Events Table that occurs within the specified time period after the vaccination. Healthcare providers are strongly encouraged to report: y Any adverse event that occurs after the administration of a vaccine licensed in the United States, whether it is or is not clear that a vaccine caused the adverse event y Vaccine administration errors A copy of the Reportable Events Table can be found on the following page, or at https://vaers.hhs.gov/resources/infoproviders.html Note: COVID-19 vaccines under an Emergency Use Authorization have additional VAERS reporting requirements. -

Vaccines and Immunization February 20-21, 2017 Berlin, Germany
Lallan Giri, J Vaccines Vaccin 2017, 8:1(Suppl) conferenceseries.com http://dx.doi.org/10.4172/2157-7560.C1.054 15th Annual Summit on Vaccines and Immunization February 20-21, 2017 Berlin, Germany Protection against bioterrorism Lallan Giri Biologics Resources LLC, USA ioterrorism involves the intentional release of dissemination of deadly biological agents in the cities and communities. Terrorism Bhas become a common and easier way of causing harms to innocent public. At present time, it has caused serious concerns and insecurity in the public domain. One of the sources or methods used by terrorists is through dissemination of deadly bacteria, viruses, and chemicals. Bioterrorism agents are found typically in nature and can be grown easily. It is also possible that they can be mutated or altered to increase their ability to cause deadly diseases causing mass casualty of human lives. Anthrax letter attacks after September 11, 2001 demonstrated the ease of using the anthrax spores as a weapon of mass destruction in a bioterrorist attack. Anthrax is lethal infectious disease caused by the spore forming Bacillus anthracis. The two major virulence factors of B. anthracis are the poly-y-glutamic acid (Y-D-PGA) capsule and exotoxin. The three components of the exotoxin, Protective Antigen (PA), Lethal Factor (LF) and Edema Factor (EF) are produced and secreted separately and form two bipartite toxins that cause massive edema and organ failure. The anti-phagocytic y-D-PGA capsule disguises the bacilli from immune surveillance by macrophages and allows unimpeded growth of the bacilli leading to anthrax disease caused by both, the capsule and toxins.