RNA-Protein Interaction Analysis of SARS-Cov-2 5'- and 3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paraspeckles: Possible Nuclear Hubs by the RNA for the RNA
BioMol Concepts, Vol. 3 (2012), pp. 415–428 • Copyright © by Walter de Gruyter • Berlin • Boston. DOI 10.1515/bmc-2012-0017 Review Paraspeckles: possible nuclear hubs by the RNA for the RNA Tetsuro Hirose 1, * and Shinichi Nakagawa 2 Introduction 1 Biomedicinal Information Research Center , National Institute of Advanced Industrial Science and Technology, The eukaryotic cell nucleus is highly compartmentalized. 2-4-7 Aomi, Koutou 135-0064, Tokyo , Japan More than 10 membraneless subnuclear organelles have 2 RNA Biology Laboratory , RIKEN Advanced Research been identifi ed (1, 2) . These so-called nuclear bodies exist Institute, 2-1 Hirosawa, Wako 351-0198 , Japan in the interchromosomal space, where they are enriched in multiple nuclear regulatory factors, such as transcription and * Corresponding author RNA-processing factors. These factors are thought to serve e-mail: [email protected] as specialized hubs for various nuclear events, including transcriptional regulation and RNA processing (3, 4) . Some nuclear bodies serve as sites for the biogenesis of macromo- Abstract lecular machineries, such as ribosomes and spliceosomes. Multiple cancer cell types show striking alterations in their The mammalian cell nucleus is a highly compartmental- nuclear body organization, including changes in the numbers, ized system in which multiple subnuclear structures, called shapes and sizes of certain nuclear bodies (5) . The structural nuclear bodies, exist in the nucleoplasmic spaces. Some of complexity and dynamics of nuclear bodies have been impli- the nuclear bodies contain specifi c long non-coding RNAs cated in the regulation of complex gene expression pathways (ncRNAs) as their components, and may serve as sites for in mammalian cells. -

Straightjacket/Α2δ3 Deregulation Is Associated with Cardiac Conduction Defects in Myotonic Dystrophy Type 1
bioRxiv preprint doi: https://doi.org/10.1101/431569; this version posted October 2, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Straightjacket/α2δ3 deregulation is associated with cardiac conduction defects in Myotonic Dystrophy type 1 Emilie Plantié1, Masayuki Nakamori2, Yoan Renaud3, Aline Huguet4, Caroline Choquet5, Cristiana Dondi1, Lucile Miquerol5, Masanori Takahashi6, Geneviève Gourdon4, Guillaume Junion1, Teresa Jagla1, Monika Zmojdzian1* and Krzysztof Jagla1* 1 GReD, CNRS UMR6293, INSERM U1103, University of Clermont Auvergne, 28, Place Henri Dunant, 63000 Clermont-Ferrand, France 2 Department of Neurology, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan 3 BYONET (www.byonet.fr) 4 Imagine Institute, Inserm UMR1163, 24, boulevard de Montparnasse, 75015 Paris, France 5 Aix-Marseille University, CNRS UMR7288, IBDM Luminy Campus Case 907, 13288 Marseille cedex 9, France 6 Department of Functional Diagnostic Science, Osaka University Graduate School of Medicine, 1-7 Yamadaoka, Suita, Osaka 565-0871, Japan • Correspondence to: Krzysztof Jagla [email protected] and Monika Zmojdzian [email protected] tel. +33 473178181; GReD, CNRS UMR6293, INSERM U1103, University of Clermont Auvergne, 28, Place Henri Dunant, 63000 Clermont-Ferrand, France 1 bioRxiv preprint doi: https://doi.org/10.1101/431569; this version posted October 2, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. ABSTRACT Cardiac conduction defects decrease life expectancy in myotonic dystrophy type 1 (DM1), a complex toxic CTG repeat disorder involving misbalance between two RNA- binding factors, MBNL1 and CELF1. -

Supplemental Table 1A. Differential Gene Expression Profile of Adehcd40l and Adehnull Treated Cells Vs Untreated Cells
Supplemental Table 1a. Differential Gene Expression Profile of AdEHCD40L and AdEHNull treated cells vs Untreated Cells Fold change Regulation Fold change Regulation ([AdEHCD40L] vs ([AdEHCD40L] ([AdEHNull] vs ([AdEHNull] vs Probe Set ID [Untreated]) vs [Untreated]) [Untreated]) [Untreated]) Gene Symbol Gene Title RefSeq Transcript ID NM_001039468 /// NM_001039469 /// NM_004954 /// 203942_s_at 2.02 down 1.00 down MARK2 MAP/microtubule affinity-regulating kinase 2 NM_017490 217985_s_at 2.09 down 1.00 down BAZ1A fibroblastbromodomain growth adjacent factor receptorto zinc finger 2 (bacteria-expressed domain, 1A kinase, keratinocyte NM_013448 /// NM_182648 growth factor receptor, craniofacial dysostosis 1, Crouzon syndrome, Pfeiffer 203638_s_at 2.10 down 1.01 down FGFR2 syndrome, Jackson-Weiss syndrome) NM_000141 /// NM_022970 1570445_a_at 2.07 down 1.01 down LOC643201 hypothetical protein LOC643201 XM_001716444 /// XM_001717933 /// XM_932161 231763_at 3.05 down 1.02 down POLR3A polymerase (RNA) III (DNA directed) polypeptide A, 155kDa NM_007055 1555368_x_at 2.08 down 1.04 down ZNF479 zinc finger protein 479 NM_033273 /// XM_001714591 /// XM_001719979 241627_x_at 2.15 down 1.05 down FLJ10357 hypothetical protein FLJ10357 NM_018071 223208_at 2.17 down 1.06 down KCTD10 potassium channel tetramerisation domain containing 10 NM_031954 219923_at 2.09 down 1.07 down TRIM45 tripartite motif-containing 45 NM_025188 242772_x_at 2.03 down 1.07 down Transcribed locus 233019_at 2.19 down 1.08 down CNOT7 CCR4-NOT transcription complex, subunit 7 NM_013354 -

Table S3a Table
Table S3a C2 KEGG Geneset Genesets enriched and upregulated in responders (FDR <0.25) Genesets enriched and upregulated in non-responders (FDR <0.25) HSA04610_COMPLEMENT_AND_COAGULATION_CASCADES HSA00970_AMINOACYL_TRNA_BIOSYNTHESIS HSA04640_HEMATOPOIETIC_CELL_LINEAGE HSA05050_DENTATORUBROPALLIDOLUYSIAN_ATROPHY HSA04060_CYTOKINE_CYTOKINE_RECEPTOR_INTERACTION HSA04514_CELL_ADHESION_MOLECULES HSA04650_NATURAL_KILLER_CELL_MEDIATED_CYTOTOXICITY HSA04630_JAK_STAT_SIGNALING_PATHWAY HSA03320_PPAR_SIGNALING_PATHWAY HSA04080_NEUROACTIVE_LIGAND_RECEPTOR_INTERACTION HSA00980_METABOLISM_OF_XENOBIOTICS_BY_CYTOCHROME_P450 HSA00071_FATTY_ACID_METABOLISM HSA04660_T_CELL_RECEPTOR_SIGNALING_PATHWAY HSA04612_ANTIGEN_PROCESSING_AND_PRESENTATION HSA04662_B_CELL_RECEPTOR_SIGNALING_PATHWAY HSA04920_ADIPOCYTOKINE_SIGNALING_PATHWAY HSA00120_BILE_ACID_BIOSYNTHESIS HSA04670_LEUKOCYTE_TRANSENDOTHELIAL_MIGRATION HSA00641_3_CHLOROACRYLIC_ACID_DEGRADATION HSA04020_CALCIUM_SIGNALING_PATHWAY HSA04940_TYPE_I_DIABETES_MELLITUS HSA04512_ECM_RECEPTOR_INTERACTION HSA00010_GLYCOLYSIS_AND_GLUCONEOGENESIS HSA02010_ABC_TRANSPORTERS_GENERAL HSA04664_FC_EPSILON_RI_SIGNALING_PATHWAY HSA04710_CIRCADIAN_RHYTHM HSA04510_FOCAL_ADHESION HSA04810_REGULATION_OF_ACTIN_CYTOSKELETON HSA00410_BETA_ALANINE_METABOLISM HSA01040_POLYUNSATURATED_FATTY_ACID_BIOSYNTHESIS HSA00532_CHONDROITIN_SULFATE_BIOSYNTHESIS HSA04620_TOLL_LIKE_RECEPTOR_SIGNALING_PATHWAY HSA04010_MAPK_SIGNALING_PATHWAY HSA00561_GLYCEROLIPID_METABOLISM HSA00053_ASCORBATE_AND_ALDARATE_METABOLISM HSA00590_ARACHIDONIC_ACID_METABOLISM -

851 Genes from Wild Peromyscus Liver Showed Significant Differential Expression Gene Symbol Genbank Description 0610006A03rik BG
851 genes from wild Peromyscus Liver showed significant differential expression Gene Symbol Genbank Description 0610006A03Rik BG084074 RIKEN cDNA 0610006A03 gene 0610010D24Rik BG069698 RIKEN cDNA 0610010D24 gene 0610012H03Rik C86087 RIKEN cDNA 0610012H03 gene 0610040D20Rik BG071645 RIKEN cDNA 0610040D20 gene 1110004F14Rik AI843901 Transcribed locus 1110005A03Rik AI836508 RIKEN cDNA 1110005A03 gene 1110008P14Rik AI413491 RIKEN cDNA 1110008P14 gene 1110017I16Rik AI413224 RIKEN cDNA 1110017I16 gene 1110031I02Rik AU043030 RIKEN cDNA 1110031I02 gene 1110032D12Rik AI846771 Transcribed locus 1110039B18Rik AI841294 RIKEN cDNA 1110039B18 gene 1110059H15Rik BG063102 RIKEN cDNA 1110059H15 gene 1110063F24Rik AI843727 RIKEN cDNA 1110063F24 gene 1110065P20Rik AI413621 RIKEN cDNA 1110065P20 gene 1500003O03Rik BG082833 RIKEN cDNA 1500003O03 gene 1600029D21Rik BG063464 RIKEN cDNA 1600029D21 gene 1700001C02Rik AI414719 RIKEN cDNA 1700001C02 gene 1700020D05Rik BG088854 RIKEN cDNA 1700020D05 gene 1700034H14Rik BG088118 RIKEN cDNA 1700034H14 gene 2210401J11Rik BG069270 RIKEN cDNA 2210401J11 gene 2310005N03Rik AI843155 RIKEN cDNA 2310005N03 gene 2410025L10Rik AI839046 RIKEN cDNA 2410025L10 gene 2610019A05Rik BG074588 RIKEN cDNA 2610019A05 gene 2610205H19Rik BG082594 RIKEN cDNA 2610205H19 gene 2700069I18Rik AI414193 RIKEN cDNA 2700069I18 gene 2700078E11Rik AI851562 RIKEN cDNA 2700078E11 gene 2810003C17Rik BG080895 RIKEN cDNA 2810003C17 gene 2900042E01Rik AI415188 RIKEN cDNA 2900042E01 gene 2900073G15Rik BG084591 RIKEN cDNA 2900073G15 gene 2900097C17Rik BG073867 -

C/EBP ∆Uorf Mice – a Genetic Model for Uorf-Mediated Translational Control in Mammals
Aus dem Max-Delbrück-Centrum für molekulare Medizin C/EBP uORF mice – a genetic model for uORF-mediated translational control in mammals Dissertation zur Erlangung des akademischen Grades doctor rerum naturalium (Dr. rer. nat.) im Fach Biologie eingereicht an der Mathematisch-Naturwissenschaftlichen Fakultät I der Humboldt-Universität zu Berlin von Dr. med. Klaus Wethmar Präsident der Humboldt-Universität zu Berlin Prof. Dr. Dr. h.c. Christoph Markschies Dekan der Mathematisch-Naturwissenschaftlichen Fakultät I Prof. Dr. Andreas Herrmann Gutachter: 1. Prof. Dr. Achim Leutz 2. Prof. Dr. Claus Scheidereit 3. Prof. Dr. Thomas Sommer Tag der mündlichen Prüfung: 28. März 2011 Table of contents Table of contents 2 Zusammenfassung 4 Abstract 5 Dedication 7 List of abbreviations 8 1 Introduction 11 1.1 Translational regulation of protein expression 11 1.1.1 Mechanisms of translational control 11 1.1.2 Translational control by upstream open reading frames 14 1.1.2.1 Variable presence of uORFs in alternative transcripts 15 1.1.2.2 Length, position and initiation codon context 15 1.1.2.3 Upstream ORFs integrate the general translational status of a cell 17 1.1.2.4 Upstream ORF-encoded peptides 18 1.1.2.5 Nonsense-mediated mRNA decay 19 1.1.2.6 Variables affecting the degree of uORF-mediated MCS repression 20 1.2 CCAAT/enhancer binding proteins 21 1.2.1 Family overview 21 1.2.2 Isoform-specific functions of C/EBP transcription factors 24 1.2.3 Upstream ORF-mediated control of C/EBP isoform expression 26 1.3 Aims of the thesis 29 2 Materials and Methods -

The Emerging Role of the RBM20 and PTBP1 Ribonucleoproteins in Heart Development and Cardiovascular Diseases
G C A T T A C G G C A T genes Review The Emerging Role of the RBM20 and PTBP1 Ribonucleoproteins in Heart Development and Cardiovascular Diseases Stefania Fochi, Pamela Lorenzi, Marilisa Galasso, Chiara Stefani , Elisabetta Trabetti, Donato Zipeto and Maria Grazia Romanelli * Department of Neurosciences, Biomedicine and Movement Sciences, Section of Biology and Genetics, University of Verona, 37134 Verona, Italy; [email protected] (S.F.); [email protected] (P.L.); [email protected] (M.G.); [email protected] (C.S.); [email protected] (E.T.); [email protected] (D.Z.) * Correspondence: [email protected]; Tel.: +39-045-802-7182 Received: 9 March 2020; Accepted: 6 April 2020; Published: 8 April 2020 Abstract: Alternative splicing is a regulatory mechanism essential for cell differentiation and tissue organization. More than 90% of human genes are regulated by alternative splicing events, which participate in cell fate determination. The general mechanisms of splicing events are well known, whereas only recently have deep-sequencing, high throughput analyses and animal models provided novel information on the network of functionally coordinated, tissue-specific, alternatively spliced exons. Heart development and cardiac tissue differentiation require thoroughly regulated splicing events. The ribonucleoprotein RBM20 is a key regulator of the alternative splicing events required for functional and structural heart properties, such as the expression of TTN isoforms. Recently, the polypyrimidine tract-binding protein PTBP1 has been demonstrated to participate with RBM20 in regulating splicing events. In this review, we summarize the updated knowledge relative to RBM20 and PTBP1 structure and molecular function; their role in alternative splicing mechanisms involved in the heart development and function; RBM20 mutations associated with idiopathic dilated cardiovascular disease (DCM); and the consequences of RBM20-altered expression or dysfunction. -

Supplemental Solier
Supplementary Figure 1. Importance of Exon numbers for transcript downregulation by CPT Numbers of down-regulated genes for four groups of comparable size genes, differing only by the number of exons. Supplementary Figure 2. CPT up-regulates the p53 signaling pathway genes A, List of the GO categories for the up-regulated genes in CPT-treated HCT116 cells (p<0.05). In bold: GO category also present for the genes that are up-regulated in CPT- treated MCF7 cells. B, List of the up-regulated genes in both CPT-treated HCT116 cells and CPT-treated MCF7 cells (CPT 4 h). C, RT-PCR showing the effect of CPT on JUN and H2AFJ transcripts. Control cells were exposed to DMSO. β2 microglobulin (β2) mRNA was used as control. Supplementary Figure 3. Down-regulation of RNA degradation-related genes after CPT treatment A, “RNA degradation” pathway from KEGG. The genes with “red stars” were down- regulated genes after CPT treatment. B, Affy Exon array data for the “CNOT” genes. The log2 difference for the “CNOT” genes expression depending on CPT treatment was normalized to the untreated controls. C, RT-PCR showing the effect of CPT on “CNOT” genes down-regulation. HCT116 cells were treated with CPT (10 µM, 20 h) and CNOT6L, CNOT2, CNOT4 and CNOT6 mRNA were analysed by RT-PCR. Control cells were exposed to DMSO. β2 microglobulin (β2) mRNA was used as control. D, CNOT6L down-regulation after CPT treatment. CNOT6L transcript was analysed by Q- PCR. Supplementary Figure 4. Down-regulation of ubiquitin-related genes after CPT treatment A, “Ubiquitin-mediated proteolysis” pathway from KEGG. -

Transcriptomic Analysis of Patients with Tetralogy of Fallot Reveals the Effect of Chronic Hypoxia on Myocardial Gene Expression
Ghorbel et al Congenital Heart Disease Transcriptomic analysis of patients with tetralogy of Fallot reveals the effect of chronic hypoxia on myocardial gene expression Mohamed T. Ghorbel, PhD, Myriam Cherif, PhD, Emma Jenkins, PhD, Amir Mokhtari, MRCS, Damien Kenny, MRCPCH, Gianni D. Angelini, FRCS, and Massimo Caputo, MD Objectives: In cyanotic patients undergoing repair of heart defects, chronic hypoxia is thought to lead to greater susceptibility to ischemia and reoxygenation injury. We sought to find an explanation to such a hypothesis by investigating the cardiac gene expression in patients with tetralogy of Fallot undergoing cardiac surgery. CHD Methods: The myocardial gene profile was investigated in right ventricular biopsy specimens obtained from 20 patients with a diagnosis of cyanotic (n ¼ 11) or acyanotic (n ¼ 9) tetralogy of Fallot undergoing surgical repair. Oligonucleotide microarray analyses were performed on the samples, and the array results were validated with Western blotting and enzyme-linked immunosorbent assay. Results: Data revealed 795 differentially expressed genes in cyanotic versus acyanotic hearts, with 198 upregu- lated and 597 downregulated. Growth/morphogenesis, remodeling, and apoptosis emerged as dominant func- tional themes for the upregulated genes and included the apoptotic gene TRAIL (tumor necrosis factor–related apoptosis-inducing ligand), the remodeling factor OPN (osteopontin), and the mitochondrial function gene COX11 (cytochrome-c oxidase 11). In contrast, transcription, mitogen-activated protein kinase signaling, and contractile machinery were the dominant functional classes for the downregulated genes, which included the calcium-handling gene NCX1 (sodium-calcium exchanger). Protein levels of COX11, NCX1, OPN, and LYZ (ly- sozyme) in the myocardium followed the same pattern obtained by means of transcriptomics. -
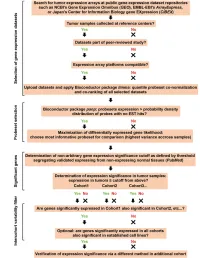
Supplementary Data
SUPPLEMENTAL INFORMATION A study restricted to chemokine receptors as well as a genome-wide transcript analysis uncovered CXCR4 as preferentially expressed in Ewing's sarcoma (Ewing's sarcoma) cells of metastatic origin (Figure 4). Transcriptome analyses showed that in addition to CXCR4, genes known to support cell motility and invasion topped the list of genes preferentially expressed in metastasis-derived cells (Figure 4D). These included kynurenine 3-monooxygenase (KMO), galectin-1 (LGALS1), gastrin-releasing peptide (GRP), procollagen C-endopeptidase enhancer (PCOLCE), and ephrin receptor B (EPHB3). KMO, a key enzyme of tryptophan catabolism, has not been linked to metastasis. Tryptophan and its catabolites, however, are involved in immune evasion by tumors, a process that can assist in tumor progression and metastasis (1). LGALS1, GRP, PCOLCE and EPHB3 have been linked to tumor progression and metastasis of several cancers (2-4). Top genes preferentially expressed in L-EDCL included genes that suppress cell motility and/or potentiate cell adhesion such as plakophilin 1 (PKP1), neuropeptide Y (NPY), or the metastasis suppressor TXNIP (5-7) (Figure 4D). Overall, L-EDCL were enriched in gene sets geared at optimizing nutrient transport and usage (Figure 4D; Supplementary Table 3), a state that may support the early stages of tumor growth. Once tumor growth outpaces nutrient and oxygen supplies, gene expression programs are usually switched to hypoxic response and neoangiogenesis, which ultimately lead to tumor egress and metastasis. Accordingly, gene sets involved in extracellular matrix remodeling, MAPK signaling, and response to hypoxia were up-regulated in M-EDCL (Figure 4D; Supplementary Table 4), consistent with their association to metastasis in other cancers (8, 9). -

Molecular Mechanism and Metabolic Function of the S
MOLECULAR MECHANISM AND METABOLIC FUNCTION OF THE S- NITROSO-COENZYME A REDUCTASE AKR1A1 by COLIN T. STOMBERSKI Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Dissertation Advisor: Jonathan S. Stamler Department of Biochemistry CASE WESTERN RESERVE UNIVERSITY May, 2019 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the dissertation of COLIN T. STOMBERSKI candidate for the degree of Doctor of Philosophy*. Committee Chair Focco van den Akker Committee Members Jonathan Stamler George Dubyak Mukesh Jain Hung-Ying Kao 03-22-2019 *We also certify that written approval has been obtained for any proprietary material contained therein TABLE OF CONTENTS Table of Contents ………………………………………………………………………… i List of Tables ……………………………………………………………………………. v List of Figures ………………………………………………………………………….. vi List of Abbreviations …………………………………………………………………… ix Acknowledgements …………………………………………………………………….. xi Abstract ………………………………………………………………………………….. 1 Foundation and Experimental Framework ……………………………………………….. 3 Chapter 1: Protein S-nitrosylation: Determinants of specificity and enzymatic regulation of S-nitrosothiol-based signaling …………………………………………….. 5 1.1 Introduction …………………………………………………………………. 6 1.2 S-nitrosothiol specificity …………………………………………………….. 8 1.2.1 Acid-base and hydrophobic motifs ……………………………… 9 1.2.2 Interaction with nitric oxide synthases ………………………….. 13 1.3 S-nitrosothiol stability and reactivity ……………………………………….. 15 1.3.1 RSNO bond chemistry ………………………………………….. 16 1.3.2 Protein SNO—thiol reaction bias ………………………………. 18 1.3.3 SNO sites do not overlap S-oxidation sites …………………….. 19 1.4 Enzymatic denitrosylation ………………………………………………….. 20 1.4.1 The thioredoxin system ………………………………………… 21 1.4.2 LMW-SNO reductases …………………………………………. 23 1.4.3 The GSNO reductase system …………………………………… 24 1.4.4 GSNOR in physiology and pathophysiology …………………… 26 i 1.4.5 The SNO-CoA reductase system ……………………………….. 31 1.5 Specificity in denitrosylation ………………………………………………. -

Molecular Basis of the Human Ribosomopathy Shwachman-Diamond Syndrome
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Apollo Advances in Biological Regulation 67 (2018) 109e127 Contents lists available at ScienceDirect Advances in Biological Regulation journal homepage: www.elsevier.com/locate/jbior Molecular basis of the human ribosomopathy Shwachman- Diamond syndrome * Alan J. Warren a, b, c, a Cambridge Institute for Medical Research, Cambridge, UK b The Department of Haematology, University of Cambridge, Cambridge, UK c Wellcome Trust-Medical Research Council Stem Cell Institute, University of Cambridge, Cambridge, UK article info abstract Article history: Mutations that target the ubiquitous process of ribosome assembly paradoxically cause Received 4 September 2017 diverse tissue-specific disorders (ribosomopathies) that are often associated with an Accepted 5 September 2017 increased risk of cancer. Ribosomes are the essential macromolecular machines that read Available online 6 September 2017 the genetic code in all cells in all kingdoms of life. Following pre-assembly in the nucleus, precursors of the large 60S and small 40S ribosomal subunits are exported to the cyto- Keywords: plasm where the final steps in maturation are completed. Here, I review the recent insights SBDS into the conserved mechanisms of ribosome assembly that have come from functional DNAJC21 eIF6 characterisation of the genes mutated in human ribosomopathies. In particular, recent Shwachman-Diamond syndrome advances in cryo-electron microscopy, coupled with genetic, biochemical and prior Ribosome structural data, have revealed that the SBDS protein that is deficient in the inherited Myelodysplastic syndromes leukaemia predisposition disorder Shwachman-Diamond syndrome couples the final step in cytoplasmic 60S ribosomal subunit maturation to a quality control assessment of the structural and functional integrity of the nascent particle.