An Important One Health Opportunity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
If Needed You Can Use Two Lines
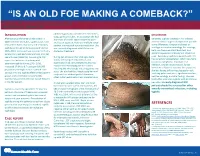
“IS AN OLD FOE MAKING A COMEBACK?” •Eyob Tadesse MD1; Samie Meskele MD1; Ankoor Biswas MD1 •Aurora Health Care, Milwaukee WI. NTRODUCTION abdomen gradually spread to her extremities, DISCUSSION I scalp, palms and soles. In association she had After being on the verge of elimination in shortness of breath, vague abdominal pain Generally, syphilis presents in HIV infected 2000 in the United States, syphilis cases have and loss of appetite, history of multiple sexual patients similar to general population yet with rebounded. Rates of primary and secondary partner, unprotected sex and prostitution. She some differences. Diagnosis is based on syphilis continued to increase overall during was recently diagnosed with HIV but not serologic test and microbiology. For serology, 2005–2013. Increases have occurred primarily started on treatment. both non treponemal antibody test, and among men, and particularly among men has specific treponemal antibody test should be used. Secondary syphilis in patients with HIV sex with men (MSM)(1). According to CDC During her admission her vital signs were has varied skin presentation, which can mimic report the incidence of primary and stable, she had pale conjunctivae, skin cutaneous lymphoma, mycobacterial secondary syphilis during 2015–2016, examination had demonstrated widespread macular and maculopapular skin lesions infection, bacillary angiomatosis, fungal increased 17.6% to 8.7 cases per 100,000 infections or Kaposi’s sarcoma. In our patient, population, the highest rate reported since involving the whole body including palms and soles. She also had thin, fragile scalp hair and she was having diffuse maculopapular rash, 1993(2). HIV and syphilis affect similar patient scalp hair loss without genital ulceration; involving palms and soles, significant hair loss, groups and co-infection is common(3). -

HIV and the SKIN • Sudden Acute Exacerbations • Treatment Failure DR
2018/08/13 KEY FEATURES • Atypical presentation of common disorders • Severe or exaggerated presentations HIV AND THE SKIN • Sudden acute exacerbations • Treatment failure DR. FREDAH MALEKA DERMATOLOGY UNIVERSITY OF PRETORIA:KALAFONG VIRAL INFECTIONS EXANTHEM OF PRIMARY HIV INFECTION • Exanthem of primary HIV infection • Acute retroviral syndrome • Herpes simplex virus (HSV) • Morbilliform rash (exanthem) : 2-4 weeks after HIV exposure • Varicella Zoster virus (VZV) • Typically generalised • Molluscum contagiosum (Poxvirus) • Pronounced on face and trunk, sparing distal extremities • Human papillomavirus (HPV) • Associated : fever, lymphadenopathy, pharyngitis • Epstein Barr virus (EBV) • DDX: drug reaction • Cytomegalovirus (CMV) • other viral infections – EBV, Enteroviruses, Hepatitis B virus 1 2018/08/13 HERPES SIMPLEX VIRUS(HSV) • Vesicular eruption due to HSV 1&2 • Primary lesion: painful, grouped vesicles on an erythematous base • HIV: attacks are more frequent and severe • : chronic, non-healing, deep ulcers, with scarring and tissue destruction • CLUE: severe pain and recurrences • DDX: syphilis, chancroid, lymphogranuloma venereum • Tzanck smear, Histology, Viral culture HSV • Treatment: Acyclovir 400mg tds 7-10 days • Alternatives: Valacyclovir and Famciclovir • In setting of treatment failure, viral isolates tested for resistance against acyclovir • Alternative drugs: Foscarnet, Cidofovir • Chronic suppressive therapy ( >8 attacks per year) 2 2018/08/13 VARICELLA • Chickenpox • Presents with erythematous papules and umbilicated -

WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/05 (2006.01) A61P 31/02 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/CA20 14/000 174 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 4 March 2014 (04.03.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 13/790,91 1 8 March 2013 (08.03.2013) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: LABORATOIRE M2 [CA/CA]; 4005-A, rue kind of regional protection available): ARIPO (BW, GH, de la Garlock, Sherbrooke, Quebec J1L 1W9 (CA). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (72) Inventors: LEMIRE, Gaetan; 6505, rue de la fougere, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Sherbrooke, Quebec JIN 3W3 (CA). -

Rheumatic Manifestations of Bartonella Infection in 2 Children MOHAMMAD J
Case Report Rheumatic Manifestations of Bartonella Infection in 2 Children MOHAMMAD J. AL-MATAR, ROSS E. PETTY, DAVID A. CABRAL, LORI B. TUCKER, BANAFSHI PEYVANDI, JULIE PRENDIVILLE, JACK FORBES, ROBYN CAIRNS, and RALPH ROTHSTEIN ABSTRACT. We describe 2 patients with very unusual rheumatological presentations presumably caused by Bartonella infection: one had myositis of proximal thigh muscles bilaterally, and the other had arthritis and skin nodules. Both patients had very high levels of antibody to Bartonella that decreased in asso- ciation with clinical improvement. Bartonella infection should be considered in the differential diag- nosis of unusual myositis or arthritis in children. (J Rheumatol 2002;29:184–6) Key Indexing Terms: MYOSITIS ARTHRITIS BARTONELLA Infection with Bartonella species has a wide range of mani- was slightly increased at 9.86 IU/l (normal 4.51–9.16), and IgA was 2.1 IU/l festations in children including cat scratch disease (regional (normal 0.2–1.0). C3 was 0.11 g/l (normal 0.77–1.43) and C4 was 0.28 (nor- mal 0.07–0.40). Antinuclear antibodies were present at a titer of 1:40, the anti- granulomatous lymphadenitis), bacillary angiomatosis, streptolysin O titer was 35 (normal < 200), and the anti-DNAase B titer was encephalitis, Parinaud’s oculoglandular syndrome, Trench 1:85 (normal). Urinalysis showed 50–100 erythrocytes and 5–10 leukocytes fever (Vincent’s angina), osteomyelitis, granulomatous per high power field. Routine cultures of urine, blood, and throat were nega- hepatitis, splenitis, pneumonitis, endocarditis, and fever of tive. Liver enzymes, electrolytes, HIV serology, cerebrospinal fluid analysis, unknown origin1-3. -

Circulatory and Lymphatic System Infections 1105
Chapter 25 | Circulatory and Lymphatic System Infections 1105 Chapter 25 Circulatory and Lymphatic System Infections Figure 25.1 Yellow fever is a viral hemorrhagic disease that can cause liver damage, resulting in jaundice (left) as well as serious and sometimes fatal complications. The virus that causes yellow fever is transmitted through the bite of a biological vector, the Aedes aegypti mosquito (right). (credit left: modification of work by Centers for Disease Control and Prevention; credit right: modification of work by James Gathany, Centers for Disease Control and Prevention) Chapter Outline 25.1 Anatomy of the Circulatory and Lymphatic Systems 25.2 Bacterial Infections of the Circulatory and Lymphatic Systems 25.3 Viral Infections of the Circulatory and Lymphatic Systems 25.4 Parasitic Infections of the Circulatory and Lymphatic Systems Introduction Yellow fever was once common in the southeastern US, with annual outbreaks of more than 25,000 infections in New Orleans in the mid-1800s.[1] In the early 20th century, efforts to eradicate the virus that causes yellow fever were successful thanks to vaccination programs and effective control (mainly through the insecticide dichlorodiphenyltrichloroethane [DDT]) of Aedes aegypti, the mosquito that serves as a vector. Today, the virus has been largely eradicated in North America. Elsewhere, efforts to contain yellow fever have been less successful. Despite mass vaccination campaigns in some regions, the risk for yellow fever epidemics is rising in dense urban cities in Africa and South America.[2] In an increasingly globalized society, yellow fever could easily make a comeback in North America, where A. aegypti is still present. -

Are Mycobacterium Drugs Effective for Treatment Resistant Lyme Disease, Tick-Borne Co-Infections, and Autoimmune Disease?
Central JSM Arthritis Bringing Excellence in Open Access Case Report *Corresponding author Richard I. Horowitz, Hudson Valley Healing Arts Center, 4232 Albany Post Road, Hyde Park, New York 12538, Are Mycobacterium Drugs USA, Tel: 845-229-8977; Fax: 845-229-8930; Email: Submitted: 15 June 2016 Effective for Treatment Accepted: 14 July 2016 Published: 16 July 2016 Resistant Lyme Disease, Tick- Copyright © 2016 Horowitz et al. Borne Co-Infections, and OPEN ACCESS Keywords Autoimmune Disease? • Lyme disease • Bartonella Richard I. Horowitz* and Phyllis R. Freeman • Tularemia Hudson Valley Healing Arts Center, USA • Behçet’s Disease/Syndrome • Rheumatoid arthritis • Dapsone Abstract • Pyrazinamide Introduction: PTLDS/chronic Lyme disease may cause disabling symptoms with • Persister bacteria associated overlapping autoimmune manifestations, with few clinically effective published treatment options. We recently reported on the successful use of a mycobacterium drug, Dapsone, for those with PTLDS. We now report on the novel use of another mycobacterium drug, pyrazinamide, (PZA), in relieving resistant symptomatology secondary to Lyme disease and associated co-infections, while decreasing autoimmune manifestations with Behçet’s syndrome. Method: Disabling multi-systemic/arthritic symptoms persisted in a Lyme patient with co-infections (Bartonella, tularemia) and overlapping rheumatoid arthritis/ Behçet’s disease, despite several rotations of classic antibiotic and DMARD regimens. Dapsone, a published treatment protocol used for Behçet’s syndrome, recently has been demonstrated to be effective in the treatment of PTLDS/chronic Lyme disease and co-infections. It was superior to prior treatment regimens in relieving some resistant chronic tick-borne/autoimmune manifestations; however, it did not effectively treat the skin lesions and ulcers secondary to Behçet’s disease, nor significantly affect the granuloma formation, joint swelling, and pain associated with Lyme, Bartonella, and RA. -

Seroprevalence of Six Pathogens Transmitted by the Ixodes Ricinus
www.nature.com/scientificreports OPEN Seroprevalence of six pathogens transmitted by the Ixodes ricinus ticks in asymptomatic individuals Received: 9 October 2018 Accepted: 7 January 2019 with HIV infection and in blood Published: xx xx xxxx donors Agnieszka Pawełczyk1,5, Małgorzata Bednarska2,5, Justyna D. Kowalska3, Beata Uszyńska- Kałuża4, Marek Radkowski1 & Renata Welc-Falęciak 2,5 The objective of our study was to estimate the seroprevalence of six pathogens transmitted by ticks in HIV-infected persons and blood donors in Poland (B. burgdorferi s.l., A. phagocytophilum, Ehrlichia spp., Babesia spp., Rickettsia spp. Bartonella henselae) to assess the frequency of exposure to such microorganisms in immunocompetent and immunocompromised individuals in endemic regions for I. ricinus ticks. Serum samples were collected from 227 HIV-infected patients and 199 blood donors. All samples were analyzed for antibodies against six tick-borne pathogens and seroprevalence rates were statistically compared between two tested group as well as age, sex and lymphocyte T CD4+ level in HIV infected patients. The seroprevalence of tick-borne infections in HIV-infected patients is higher than that of the healthy population in Poland, although no association between serological status of patients and lymphocyte CD4+ T cell level has been observed. The frequency of tick-borne coinfections and doubtful results of serological tests were signifcantly higher in HIV-positive individuals. In Poland, the possibility of tick-borne diseases transmission with blood is rather negligible. Recently the experts of the Center for Disease Control and Prevention’s have summarized the alarming increase in the number of vector-borne disease cases reported in the United States and territories from 2004 to 20161. -

Bacillary Angiomatosis
-------------------------------'--=- Bacillary angiomatosis The first case reported in South Africa G. R. LEVY, S.NAYLER Abstract A 28-year-old white man, positive for HIV, who was admitted to hospital for pneUDlonia, had for 2 months had several fluctuating cutaneous purple nodules on his legs and abdomen. Cultures oftwo lesions were negative, but light and electron microscopy showed organisms characteristic of bacillary angiomatosis. The patieilt responded well to therapy with erythromycin. This is the first reported case of bacillary angiomatosis in South Africa. S AIrMed J 1993; 83: 855-856. acillary angiomatosis, first described in the USA in 1983,' is an infectious disease that affects FIG. 1. B immunocompromised hosts. It is characterised by Healing ulcerated lesion on right thigh, 4 days after pseudoneoplastic angioproliferative cutaneous lesions erythromycin was started. Biopsy wound left upper and may also involve internal organs. 2 It is caused by a quadrant. pleomorphic bacillus, probably Rochalimaea henselae. 3 We report on the first documented South African patient who presented with skin lesions, unusual for this pus from the ulcerated lesion was negative for bacteria ~ disease. As the organism is difficult to culture and the and fungi. disease responds well to treatment, it is important for clinicians to be aware of the condition and to do biop~ sies to verify the diagnosis. Skin biopsies The ulcerated nodule on the thigh and a subcutaneous nodule on the abdomen were biopsied 2 days after Case report antibiotic therapy was initiated. The epidermis was ulcerated in the thigh lesion and slightly atrophic in the The patient was a 28~year~0Id white homosexual man other. -

Chronic Lyme Disease and Co-Infections: Differential Diagnosis
Send Orders of Reprints at [email protected] 158 The Open Neurology Journal, 2012, 6, (Suppl 1-M10) 158-178 Open Access Chronic Lyme Disease and Co-infections: Differential Diagnosis Walter Berghoff* Practice of Internal Medicine, Rheinbach, 53359, Germany Abstract: In Lyme disease concurrent infections frequently occur. The clinical and pathological impact of co-infections was first recognized in the 1990th, i.e. approximately ten years after the discovery of Lyme disease. Their pathological synergism can exacerbate Lyme disease or induce similar disease manifestations. Co-infecting agents can be transmitted together with Borrelia burgdorferi by tick bite resulting in multiple infections but a fraction of co-infections occur inde- pendently of tick bite. Clinically relevant co-infections are caused by Bartonella species, Yersinia enterocolitica, Chlamy- dophila pneumoniae, Chlamydia trachomatis, and Mycoplasma pneumoniae. In contrast to the USA, human granulocytic anaplasmosis (HGA) and babesiosis are not of major importance in Europe. Infections caused by these pathogens in pa- tients not infected by Borrelia burgdorferi can result in clinical symptoms similar to those occurring in Lyme disease. This applies particularly to infections caused by Bartonella henselae, Yersinia enterocolitica, and Mycoplasma pneumo- niae. Chlamydia trachomatis primarily causes polyarthritis. Chlamydophila pneumoniae not only causes arthritis but also affects the nervous system and the heart, which renders the differential diagnosis difficult. The diagnosis is even more complex when co-infections occur in association with Lyme disease. Treatment recommendations are based on individual expert opinions. In antibiotic therapy, the use of third generation cephalosporins should only be considered in cases of Lyme disease. The same applies to carbapenems, which however are used occasionally in infections caused by Yersinia enterocolitica. -

Bartonella Spp. - a Chance to Establish One Health Concepts in Veterinary and Human Medicine Yvonne Regier1, Fiona O’Rourke1 and Volkhard A
Regier et al. Parasites & Vectors (2016) 9:261 DOI 10.1186/s13071-016-1546-x REVIEW Open Access Bartonella spp. - a chance to establish One Health concepts in veterinary and human medicine Yvonne Regier1, Fiona O’Rourke1 and Volkhard A. J. Kempf1* Abstract Infectious diseases remain a remarkable health threat for humans and animals. In the past, the epidemiology, etiology and pathology of infectious agents affecting humans and animals have mostly been investigated in separate studies. However, it is evident, that combined approaches are needed to understand geographical distribution, transmission and infection biology of “zoonotic agents”. The genus Bartonella represents a congenial example of the synergistic benefits that can arise from such combined approaches: Bartonella spp. infect a broad variety of animals, are linked with a constantly increasing number of human diseases and are transmitted via arthropod vectors. As a result, the genus Bartonella is predestined to play a pivotal role in establishing a One Health concept combining veterinary and human medicine. Keywords: Ticks, Fleas, Lice, Cats, Dogs, Humans, Infection, Transmission, Zoonosis Background between medical, veterinary and environmental re- The threat of infectious diseases to mankind has never searchers as well as public health officials for the early been greater than today. For the first time, political detection of health hazards affecting both humans and leaders of the 41st “G7 summit” in Schloss Elmau/ animals and to fight them on multiple levels. The genus Germany on June 7–8, 2015, set the topic “global health” Bartonella represents a prototypical example for zoo- (including infectious diseases) as one of the key issues notic pathogens as Bartonella species are infectious on their agenda. -

Serological Cross-Reactions Between Bartonella Quintana, Bartonella Henselae, and Coxiella Burnetii
JOURNAL OF CLINICAL MICROBIOLOGY, Sept. 1996, p. 2270–2274 Vol. 34, No. 9 0095-1137/96/$04.0010 Copyright q 1996, American Society for Microbiology Serological Cross-Reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii BERNARD LA SCOLA AND DIDIER RAOULT* Unite´ des Rickettsies, Faculte´deMe´decine, Centre National de la Recherche Scientifique, 13385 Marseille Cedex 05, France Received 20 March 1996/Returned for modification 26 April 1996/Accepted 21 June 1996 The clinical manifestations of Q fever and bartonelloses can be confused, especially in cases of infectious endocarditis. Differential diagnosis of the diseases is important because the treatments required for Q fever and bartonelloses are different. Laboratory confirmation of a suspected case of either Q fever or bartonelloses is most commonly made by antibody estimation with an indirect immunofluorescence assay. With an indirect immunofluorescence assay, 258 serum samples from patients with Q fever were tested against Bartonella henselae and Bartonella quintana antigens, and 77 serum samples from patients with infection by Bartonella sp. were tested against Coxiella burnetii antigen. Cross-reactivity was observed: more than 50% of the chronic Q fever patients tested had antibodies which reacted against B. henselae antigen to a significant level. This cross-reaction was confirmed by a cross-adsorption study and protein immunoblotting. However, because the levels of specific antibody titers in cases of Bartonella endocarditis are typically extremely high, low-level cross-reaction between C. burnetii antibodies and B. henselae antigen in cases of Q fever endocarditis should not lead to misdiagnosis, provided serology testing for both agents is performed. Coxiella burnetii, is the etiologic agent of Q fever. -

Introduction to Infectious Disease Epidemiology (Nelson)
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this site. Copyright 2007, The Johns Hopkins University and Kenrad Nelson. All rights reserved. Use of these materials permitted only in accordance with license rights granted. Materials provided “AS IS”; no representations or warranties provided. User assumes all responsibility for use, and all liability related thereto, and must independently review all materials for accuracy and efficacy. May contain materials owned by others. User is responsible for obtaining permissions for use from third parties as needed. Introduction to Infectious Disease Epidemiology Kenrad Nelson, MD Johns Hopkins University Section A History of Infectious Diseases in the 20th Century Crude Death Rate* for Infectious Diseases: U.S., 1906–’96 40 states have health departments *Per 100,000 population per year §American Water Works Association. (1973). Water chlorination principles and practices: AWWA manual M20. Denver, Colorado. Adapted by CTLT from Armstrong, G. L., Conn, L. A., Pinner, R. W. (1999). Trends in infectious disease mortality in the United States during the 20th century. Journal of the American Medical Association, 281, 61–66. 4 Crude Death Rate* for Infectious Diseases: U.S., 1906–’96 First continuous municipal use of chlorine in water in U.S.§ *Per 100,000 population per year §American Water Works Association. (1973). Water chlorination principles and practices: AWWA manual M20. Denver, Colorado. Adapted by CTLT from Armstrong, G. L., Conn, L. A., Pinner, R. W. (1999). Trends in infectious disease mortality in the United States during the 20th century.