Circulatory and Lymphatic System Infections 1105
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gas Gangrene Infection of the Eyes and Orbits
Br J Ophthalmol: first published as 10.1136/bjo.69.2.143 on 1 February 1985. Downloaded from British Journal of Ophthalmology, 1985, 69, 143-148 Gas gangrene infection of the eyes and orbits GERARD W CROCK,' WILSON J HERIOT,' PATTABIRAMAN JANAKIRAMAN,' AND JOHN M WEINER2 From the 'Department of Ophthalmology, University ofMelbourne, and the2C H Greer Pathology Laboratory, the Royal Victorian Eye and Ear Hospital, East Melbourne, Australia SUMMARY The literature on Clostridium perfringens infections is reviewed up to 1983. An additional case is reported with bilateral clostridial infections of the eye and orbit. One eye followed the classical course of relentless panophthalmitis, amaurosis, and orbital cellulitis ending in enucleation. The second eye contained intracameral mud and gas bubbles that were removed by vitrectomy instrumentation. Subsequent removal of the toxic cataract resulted in a final aided visual acuity of 6/18, N8. This is the third report of a retained globe, and we believe the only known case where the patient was left with useful vision. Clostridium perfringens is a ubiquitous Gram- arms, chest, and abdomen. He was admitted to a positive bacillus found in soil and bowel flora. It is the general hospital, where he was examined under most common of four clostridia species identified in anaesthesia, and his injuries were attended to. copyright. cases of gas gangrene in man.' All species are obligate Ocular findings. The right cornea and anterior anaerobes and are usually saprophytic rather than chamber were intact. There was a scleral laceration pathogenic. Clostridium perfringens is a feared con- over the superonasal area of the pars plana with taminant of limb injuries and may result in death due vitreous prolapse. -

Melioidosis: an Emerging Infectious Disease
Review Article www.jpgmonline.com Melioidosis: An emerging infectious disease Raja NS, Ahmed MZ,* Singh NN** Department of Medical ABSTRACT Microbiology, University of Malaya Medical Center, Kuala Lumpur, Infectious diseases account for a third of all the deaths in the developing world. Achievements in understanding Malaysia, *St. the basic microbiology, pathogenesis, host defenses and expanded epidemiology of infectious diseases have Bartholomew’s Hospital, resulted in better management and reduced mortality. However, an emerging infectious disease, melioidosis, West Smithfield, London, is becoming endemic in the tropical regions of the world and is spreading to non-endemic areas. This article UK and **School of highlights the current understanding of melioidosis including advances in diagnosis, treatment and prevention. Biosciences, Cardiff Better understanding of melioidosis is essential, as it is life-threatening and if untreated, patients can succumb University, Cardiff, UK to it. Our sources include a literature review, information from international consensus meetings on melioidosis Correspondence: and ongoing discussions within the medical and scientific community. N. S. Raja, E-mail: [email protected] Received : 21-2-2005 Review completed : 20-3-2005 Accepted : 30-5-2005 PubMed ID : 16006713 KEY WORDS: Melioidosis, Burkholderia pseudomallei, Infection J Postgrad Med 2005;51:140-5 he name melioidosis [also known as Whitmore dis- in returning travellers to Europe from endemic areas.[14] The T ease] is taken from the Greek word ‘melis’ meaning geographic area of the prevalence of the organism is bound to distemper of asses and ‘eidos’ meaning resembles glanders. increase as the awareness increases. Melioidosis is a zoonotic disease caused by Pseudomonas pseudomallei [now known as Burkholderia pseudomallei], a B. -
If Needed You Can Use Two Lines
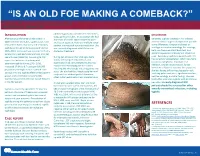
“IS AN OLD FOE MAKING A COMEBACK?” •Eyob Tadesse MD1; Samie Meskele MD1; Ankoor Biswas MD1 •Aurora Health Care, Milwaukee WI. NTRODUCTION abdomen gradually spread to her extremities, DISCUSSION I scalp, palms and soles. In association she had After being on the verge of elimination in shortness of breath, vague abdominal pain Generally, syphilis presents in HIV infected 2000 in the United States, syphilis cases have and loss of appetite, history of multiple sexual patients similar to general population yet with rebounded. Rates of primary and secondary partner, unprotected sex and prostitution. She some differences. Diagnosis is based on syphilis continued to increase overall during was recently diagnosed with HIV but not serologic test and microbiology. For serology, 2005–2013. Increases have occurred primarily started on treatment. both non treponemal antibody test, and among men, and particularly among men has specific treponemal antibody test should be used. Secondary syphilis in patients with HIV sex with men (MSM)(1). According to CDC During her admission her vital signs were has varied skin presentation, which can mimic report the incidence of primary and stable, she had pale conjunctivae, skin cutaneous lymphoma, mycobacterial secondary syphilis during 2015–2016, examination had demonstrated widespread macular and maculopapular skin lesions infection, bacillary angiomatosis, fungal increased 17.6% to 8.7 cases per 100,000 infections or Kaposi’s sarcoma. In our patient, population, the highest rate reported since involving the whole body including palms and soles. She also had thin, fragile scalp hair and she was having diffuse maculopapular rash, 1993(2). HIV and syphilis affect similar patient scalp hair loss without genital ulceration; involving palms and soles, significant hair loss, groups and co-infection is common(3). -

Fournier's Gangrene Caused by Listeria Monocytogenes As
CASE REPORT Fournier’s gangrene caused by Listeria monocytogenes as the primary organism Sayaka Asahata MD1, Yuji Hirai MD PhD1, Yusuke Ainoda MD PhD1, Takahiro Fujita MD1, Yumiko Okada DVM PhD2, Ken Kikuchi MD PhD1 S Asahata, Y Hirai, Y Ainoda, T Fujita, Y Okada, K Kikuchi. Une gangrène de Fournier causée par le Listeria Fournier’s gangrene caused by Listeria monocytogenes as the monocytogenes comme organisme primaire primary organism. Can J Infect Dis Med Microbiol 2015;26(1):44-46. Un homme de 70 ans ayant des antécédents de cancer de la langue s’est présenté avec une gangrène de Fournier causée par un Listeria A 70-year-old man with a history of tongue cancer presented with monocytogenes de sérotype 4b. Le débridement chirurgical a révélé un Fournier’s gangrene caused by Listeria monocytogenes serotype 4b. adénocarcinome rectal non diagnostiqué. Le patient n’avait pas Surgical debridement revealed undiagnosed rectal adenocarcinoma. d’antécédents alimentaires ou de voyage apparents, mais a déclaré The patient did not have an apparent dietary or travel history but consommer des sashimis (poisson cru) tous les jours. reported daily consumption of sashimi (raw fish). L’âge avancé et l’immunodéficience causée par l’adénocarcinome rec- Old age and immunodeficiency due to rectal adenocarcinoma may tal ont peut-être favorisé l’invasion directe du L monocytogenes par la have supported the direct invasion of L monocytogenes from the tumeur. Il s’agit du premier cas déclaré de gangrène de Fournier tumour. The present article describes the first reported case of attribuable au L monocytogenes. Les auteurs proposent d’inclure la con- Fournier’s gangrene caused by L monocytogenes. -

29 Assessing the Cardiovascular and Lymphatic Systems
29 Assessing the Cardiovascular and Lymphatic Systems LEARNING OUTCOMES 1. Describe the anatomy, physiology, and functions of the 5. Explain techniques used to assess cardiovascular and cardiovascular and lymphatic systems. lymphatic structure and function. 2. Describe normal variations in cardiovascular assessment 6. Identify manifestations of impaired cardiovascular structure findings for the older adult. and functions. 3. Give examples of genetic disorders of the cardiovascular system. 4. Identify specific topics for consideration during a health history assessment interview of the patient with cardiovascu- lar or lymphatic disorders. CLINICAL COMPETENCIES 1. Complete a health history for patients having alterations in 3. Assess an ECG strip and identify normal rhythm and cardiac the structure and functions of the cardiovascular or lymphatic events and abnormal cardiac rhythm. systems. 4. Monitor the results of diagnostic tests and communicate 2. Conduct and document a physical assessment of cardiovas- abnormal findings within the interprofessional team. cular and lymphatic status. MAJOR CHAPTER CONCEPTS • Correct structure and function of the cardiovascular and • Manifestations of dysfunction, injury, and disorders affecting lymphatic systems are vital to the transport of oxygen and the cardiovascular and lymphatic systems may be detected carbon dioxide throughout the body and for the return of during a general health assessment as well as during focused excess tissue fluids back to the bloodstream. cardiovascular and lymphatic system -

Kellie ID Emergencies.Pptx
4/24/11 ID Alert! recognizing rapidly fatal infections Susan M. Kellie, MD, MPH Professor of Medicine Division of Infectious Diseases, UNMSOM Hospital Epidemiologist UNMHSC and NMVAHCS Fever and…. Rash and altered mental status Rash Muscle pain Lymphadenopathy Hypotension Shortness of breath Recent travel Abdominal pain and diarrhea Case 1. The cross-country trucker A 30 year-old trucker driving from Oklahoma to California is hospitalized in Deming with fever and headache He is treated with broad-spectrum antibiotics, but deteriorates with obtundation, low platelet count, and a centrifugal petechial rash and is transferred to UNMH 1 4/24/11 What is your diagnosis? What is the differential diagnosis of fever and headache with petechial rash? (in the US) Tickborne rickettsioses ◦ RMSF Bacteria ◦ Neisseria meningitidis Key diagnosis in this case: “doxycycline deficiency” Key vector-borne rickettsioses treated with doxycycline: RMSF-case-fatality 5-10% ◦ Fever, nausea, vomiting, myalgia, anorexia and headache ◦ Maculopapular rash progresses to petechial after 2-4 days of fever ◦ Occasionally without rash Human granulocytotropic anaplasmosis (HGA): case-fatality<1% Human monocytotropic ehrlichiosis (HME): case fatality 2-3% 2 4/24/11 Lab clues in rickettsioses The total white blood cell (WBC) count is typicallynormal in patients with RMSF, but increased numbers of immature bands are generally observed. Thrombocytopenia, mild elevations in hepatic transaminases, and hyponatremia might be observed with RMSF whereas leukopenia -

BD-CS-057, REV 0 | AUGUST 2017 | Page 1
EXPLIFY RESPIRATORY PATHOGENS BY NEXT GENERATION SEQUENCING Limitations Negative results do not rule out viral, bacterial, or fungal infections. Targeted, PCR-based tests are generally more sensitive and are preferred when specific pathogens are suspected, especially for DNA viruses (Adenovirus, CMV, HHV6, HSV, and VZV), mycobacteria, and fungi. The analytical sensitivity of this test depends on the cellularity of the sample and the concentration of all microbes present. Analytical sensitivity is assessed using Internal Controls that are added to each sample. Sequencing data for Internal Controls is quantified. Samples with Internal Control values below the validated minimum may have reduced analytical sensitivity or contain inhibitors and are reported as ‘Reduced Analytical Sensitivity’. Additional respiratory pathogens to those reported cannot be excluded in samples with ‘Reduced Analytical Sensitivity’. Due to the complexity of next generation sequencing methodologies, there may be a risk of false-positive results. Contamination with organisms from the upper respiratory tract during specimen collection can also occur. The detection of viral, bacterial, and fungal nucleic acid does not imply organisms causing invasive infection. Results from this test need to be interpreted in conjunction with the clinical history, results of other laboratory tests, epidemiologic information, and other available data. Confirmation of positive results by an alternate method may be indicated in select cases. Validated Organisms BACTERIA Achromobacter -

Cutaneous Manifestations of HIV Infection Carrie L
Chapter Title Cutaneous Manifestations of HIV Infection Carrie L. Kovarik, MD Addy Kekitiinwa, MB, ChB Heidi Schwarzwald, MD, MPH Objectives Table 1. Cutaneous manifestations of HIV 1. Review the most common cutaneous Cause Manifestations manifestations of human immunodeficiency Neoplasia Kaposi sarcoma virus (HIV) infection. Lymphoma 2. Describe the methods of diagnosis and treatment Squamous cell carcinoma for each cutaneous disease. Infectious Herpes zoster Herpes simplex virus infections Superficial fungal infections Key Points Angular cheilitis 1. Cutaneous lesions are often the first Chancroid manifestation of HIV noted by patients and Cryptococcus Histoplasmosis health professionals. Human papillomavirus (verruca vulgaris, 2. Cutaneous lesions occur frequently in both adults verruca plana, condyloma) and children infected with HIV. Impetigo 3. Diagnosis of several mucocutaneous diseases Lymphogranuloma venereum in the setting of HIV will allow appropriate Molluscum contagiosum treatment and prevention of complications. Syphilis Furunculosis 4. Prompt diagnosis and treatment of cutaneous Folliculitis manifestations can prevent complications and Pyomyositis improve quality of life for HIV-infected persons. Other Pruritic papular eruption Seborrheic dermatitis Overview Drug eruption Vasculitis Many people with human immunodeficiency virus Psoriasis (HIV) infection develop cutaneous lesions. The risk of Hyperpigmentation developing cutaneous manifestations increases with Photodermatitis disease progression. As immunosuppression increases, Atopic Dermatitis patients may develop multiple skin diseases at once, Hair changes atypical-appearing skin lesions, or diseases that are refractory to standard treatment. Skin conditions that have been associated with HIV infection are listed in Clinical staging is useful in the initial assessment of a Table 1. patient, at the time the patient enters into long-term HIV care, and for monitoring a patient’s disease progression. -

Diagnostic Code Descriptions (ICD9)
INFECTIONS AND PARASITIC DISEASES INTESTINAL AND INFECTIOUS DISEASES (001 – 009.3) 001 CHOLERA 001.0 DUE TO VIBRIO CHOLERAE 001.1 DUE TO VIBRIO CHOLERAE EL TOR 001.9 UNSPECIFIED 002 TYPHOID AND PARATYPHOID FEVERS 002.0 TYPHOID FEVER 002.1 PARATYPHOID FEVER 'A' 002.2 PARATYPHOID FEVER 'B' 002.3 PARATYPHOID FEVER 'C' 002.9 PARATYPHOID FEVER, UNSPECIFIED 003 OTHER SALMONELLA INFECTIONS 003.0 SALMONELLA GASTROENTERITIS 003.1 SALMONELLA SEPTICAEMIA 003.2 LOCALIZED SALMONELLA INFECTIONS 003.8 OTHER 003.9 UNSPECIFIED 004 SHIGELLOSIS 004.0 SHIGELLA DYSENTERIAE 004.1 SHIGELLA FLEXNERI 004.2 SHIGELLA BOYDII 004.3 SHIGELLA SONNEI 004.8 OTHER 004.9 UNSPECIFIED 005 OTHER FOOD POISONING (BACTERIAL) 005.0 STAPHYLOCOCCAL FOOD POISONING 005.1 BOTULISM 005.2 FOOD POISONING DUE TO CLOSTRIDIUM PERFRINGENS (CL.WELCHII) 005.3 FOOD POISONING DUE TO OTHER CLOSTRIDIA 005.4 FOOD POISONING DUE TO VIBRIO PARAHAEMOLYTICUS 005.8 OTHER BACTERIAL FOOD POISONING 005.9 FOOD POISONING, UNSPECIFIED 006 AMOEBIASIS 006.0 ACUTE AMOEBIC DYSENTERY WITHOUT MENTION OF ABSCESS 006.1 CHRONIC INTESTINAL AMOEBIASIS WITHOUT MENTION OF ABSCESS 006.2 AMOEBIC NONDYSENTERIC COLITIS 006.3 AMOEBIC LIVER ABSCESS 006.4 AMOEBIC LUNG ABSCESS 006.5 AMOEBIC BRAIN ABSCESS 006.6 AMOEBIC SKIN ULCERATION 006.8 AMOEBIC INFECTION OF OTHER SITES 006.9 AMOEBIASIS, UNSPECIFIED 007 OTHER PROTOZOAL INTESTINAL DISEASES 007.0 BALANTIDIASIS 007.1 GIARDIASIS 007.2 COCCIDIOSIS 007.3 INTESTINAL TRICHOMONIASIS 007.8 OTHER PROTOZOAL INTESTINAL DISEASES 007.9 UNSPECIFIED 008 INTESTINAL INFECTIONS DUE TO OTHER ORGANISMS -

HIV and the SKIN • Sudden Acute Exacerbations • Treatment Failure DR
2018/08/13 KEY FEATURES • Atypical presentation of common disorders • Severe or exaggerated presentations HIV AND THE SKIN • Sudden acute exacerbations • Treatment failure DR. FREDAH MALEKA DERMATOLOGY UNIVERSITY OF PRETORIA:KALAFONG VIRAL INFECTIONS EXANTHEM OF PRIMARY HIV INFECTION • Exanthem of primary HIV infection • Acute retroviral syndrome • Herpes simplex virus (HSV) • Morbilliform rash (exanthem) : 2-4 weeks after HIV exposure • Varicella Zoster virus (VZV) • Typically generalised • Molluscum contagiosum (Poxvirus) • Pronounced on face and trunk, sparing distal extremities • Human papillomavirus (HPV) • Associated : fever, lymphadenopathy, pharyngitis • Epstein Barr virus (EBV) • DDX: drug reaction • Cytomegalovirus (CMV) • other viral infections – EBV, Enteroviruses, Hepatitis B virus 1 2018/08/13 HERPES SIMPLEX VIRUS(HSV) • Vesicular eruption due to HSV 1&2 • Primary lesion: painful, grouped vesicles on an erythematous base • HIV: attacks are more frequent and severe • : chronic, non-healing, deep ulcers, with scarring and tissue destruction • CLUE: severe pain and recurrences • DDX: syphilis, chancroid, lymphogranuloma venereum • Tzanck smear, Histology, Viral culture HSV • Treatment: Acyclovir 400mg tds 7-10 days • Alternatives: Valacyclovir and Famciclovir • In setting of treatment failure, viral isolates tested for resistance against acyclovir • Alternative drugs: Foscarnet, Cidofovir • Chronic suppressive therapy ( >8 attacks per year) 2 2018/08/13 VARICELLA • Chickenpox • Presents with erythematous papules and umbilicated -

Studies on Oral Colonization of Periodontopathogenic Bacterium
Studies on oral colonization of periodontopathogenic bacterium Eikenella corrodens 㸦ṑ࿘ཎᛶ⣽⳦ (LNHQHOODFRUURGHQV ࡢཱྀ⭍ෆᐃ╔㛵ࡍࡿ◊✲㸧 㻌 㻌 FARIHA JASIN MANSUR 2017 㻌 㻌 DEDICATED TO MY BELOVED PARENTS CONTENTS CONTENTS…………………………………………………………. 1 LIST OF ABBREVIATIONS ……………………………………. 2 CHAPTER 1: GENERAL INTRODUCTION …………………………………... 4 CHAPTER 2 .………………………………………………………. 11 2.1 ABSTRACT ……………………………………………………. 12 2.2 INTRODUCTION ……………………………………………... 13 2.3 MATERIALS AND METHODS ……………………………… 16 2.4 RESULTS AND DISCUSSION ……………………………….. 21 CHAPTER 3 ………………………………………………………... 31 3.1 ABSTRACT …………………………………………………….. 32 3.2 INTRODUCTION ……………………………………………… 33 3.3 MATERIALS AND METHODS ………………………………. 35 3.4 RESULTS AND DISCUSSION ………………………………... 38 CHAPTER 4: GENERAL CONCLUSION ………………………………………... 44 SUMMARY ………………………………………………………….. 50 JAPANESE SUMMARY ……………………………………………. 52 ACKNOWLEDGEMENTS …………………………………………. 54 REFERENCES ……………………………………………………….. 56 LIST OF PUBLICATIONS ………………………………………….. 65 㻝㻌 㻌 LIST OF ABBREVIATIONS CE Cell envelope GalNAc㻌㻌㻌㻌㻌㻌 N-acetyl-D-galactosamine g Gram g/L Gram/litre HA㻌㻌㻌㻌㻌㻌㻌㻌 Hemagglutination ∆hlyA hlyA-deficient strain H hour IL Interleukin IPTG Isopropyl β-D-1-thiogalactopyranoside LB㻌 㻌 㻌 㻌 Luria broth M Molar mM Milimolar min Minute mL Mililitre mg/mL Milligram/mililitre NaCl Sodium chloride ORF Open reading frame PBS Phosphate-buffered saline㻌 PCR Polymerase chain reaction pH㻌㻌 㻌 㻌㻌㻌㻌㻌㻌㻌Potential of hydrogen SDS–PAGE Sodium dodecyl sulfate polyacrylamide gel electrophoresis TSB Tryptic soy broth 㻞㻌 㻌 μL Microlitre μM Micromolar -

CD Alert Monthly Newsletter of National Centre for Disease Control, Directorate General of Health Services, Government of India
CD Alert Monthly Newsletter of National Centre for Disease Control, Directorate General of Health Services, Government of India May - July 2009 Vol. 13 : No. 1 SCRUB TYPHUS & OTHER RICKETTSIOSES it lacks lipopolysaccharide and peptidoglycan RICKETTSIAL DISEASES and does not have an outer slime layer. It is These are the diseases caused by rickettsiae endowed with a major surface protein (56kDa) which are small, gram negative bacilli adapted and some minor surface protein (110, 80, 46, to obligate intracellular parasitism, and 43, 39, 35, 25 and 25kDa). There are transmitted by arthropod vectors. These considerable differences in virulence and organisms are primarily parasites of arthropods antigen composition among individual strains such as lice, fleas, ticks and mites, in which of O.tsutsugamushi. O.tsutsugamushi has they are found in the alimentary canal. In many serotypes (Karp, Gillian, Kato and vertebrates, including humans, they infect the Kawazaki). vascular endothelium and reticuloendothelial GLOBAL SCENARIO cells. Commonly known rickettsial disease is Scrub Typhus. Geographic distribution of the disease occurs within an area of about 13 million km2 including- The family Rickettsiaeceae currently comprises Afghanistan and Pakistan to the west; Russia of three genera – Rickettsia, Orientia and to the north; Korea and Japan to the northeast; Ehrlichia which appear to have descended Indonesia, Papua New Guinea, and northern from a common ancestor. Former members Australia to the south; and some smaller of the family, Coxiella burnetii, which causes islands in the western Pacific. It was Q fever and Rochalimaea quintana causing first observed in Japan where it was found to trench fever have been excluded because the be transmitted by mites.