Diagnostic Code Descriptions (ICD9)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Official Nh Dhhs Health Alert
THIS IS AN OFFICIAL NH DHHS HEALTH ALERT Distributed by the NH Health Alert Network [email protected] May 18, 2018, 1300 EDT (1:00 PM EDT) NH-HAN 20180518 Tickborne Diseases in New Hampshire Key Points and Recommendations: 1. Blacklegged ticks transmit at least five different infections in New Hampshire (NH): Lyme disease, Anaplasma, Babesia, Powassan virus, and Borrelia miyamotoi. 2. NH has one of the highest rates of Lyme disease in the nation, and 50-60% of blacklegged ticks sampled from across NH have been found to be infected with Borrelia burgdorferi, the bacterium that causes Lyme disease. 3. NH has experienced a significant increase in human cases of anaplasmosis, with cases more than doubling from 2016 to 2017. The reason for the increase is unknown at this time. 4. The number of new cases of babesiosis also increased in 2017; because Babesia can be transmitted through blood transfusions in addition to tick bites, providers should ask patients with suspected babesiosis whether they have donated blood or received a blood transfusion. 5. Powassan is a newer tickborne disease which has been identified in three NH residents during past seasons in 2013, 2016 and 2017. While uncommon, Powassan can cause a debilitating neurological illness, so providers should maintain an index of suspicion for patients presenting with an unexplained meningoencephalitis. 6. Borrelia miyamotoi infection usually presents with a nonspecific febrile illness similar to other tickborne diseases like anaplasmosis, and has recently been identified in one NH resident. Tests for Lyme disease do not reliably detect Borrelia miyamotoi, so providers should consider specific testing for Borrelia miyamotoi (see Attachment 1) and other pathogens if testing for Lyme disease is negative but a tickborne disease is still suspected. -
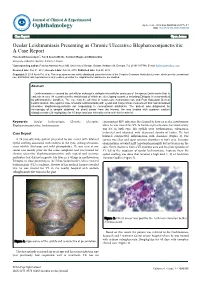
Ocular Leishmaniasis Presenting As Chronic
perim Ex en l & ta a l ic O p in l h t C h f Journal of Clinical & Experimental a o l m l a o n l r o g u Ayele et al., J Clin Exp Ophthalmol 2015, 6:1 y o J Ophthalmology ISSN: 2155-9570 DOI: 10.4172/2155-9570.1000395 Case Report Open Access Ocular Leishmaniasis Presenting as Chronic Ulcerative Blepharoconjunctivitis: A Case Report Fisseha Admassu Ayele*, Yared Assefa Wolde, Tesfalem Hagos and Ermias Diro University of Gondar, Gondar, Amhara, Ethiopia *Corresponding author: Fisseha Admassu Ayele MD, University of Gondar, Gondar, Amhara 196, Ethiopia, Tel: 251911197786; E-mail: [email protected] Received date: Dec 01, 2014, Accepted date: Feb 02, 2015, Published date: Feb 05, 2015 Copyright: © 2015 Ayele FH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Leishmaniasis is caused by unicellular eukaryotic obligate intracellular protozoa of the genus Leishmania that is endemic in over 98 countries in the world-most of which are developing countries including Ethiopia. It is transmitted by phlebotomine sandflies. The eye may be affected in cutaneous, mucocutaneous and Post Kala-Azar Dermal Leishmaniasis. We report a case of ocular leishmaniasis with eyelid and conjunctival involvement that had simulated ulcerative blepharoconjunctivitis not responding to conventional antibiotics. The patient was diagnosed by microscopy of a sample obtained via direct smear from the lesions. He was treated with systemic sodium stibogluconate (20 mg/kg/day) for 45 days and was clinically cured with this treatment. -

Molecular Evidence of Novel Spotted Fever Group Rickettsia
pathogens Article Molecular Evidence of Novel Spotted Fever Group Rickettsia Species in Amblyomma albolimbatum Ticks from the Shingleback Skink (Tiliqua rugosa) in Southern Western Australia Mythili Tadepalli 1, Gemma Vincent 1, Sze Fui Hii 1, Simon Watharow 2, Stephen Graves 1,3 and John Stenos 1,* 1 Australian Rickettsial Reference Laboratory, University Hospital Geelong, Geelong 3220, Australia; [email protected] (M.T.); [email protected] (G.V.); [email protected] (S.F.H.); [email protected] (S.G.) 2 Reptile Victoria Inc., Melbourne 3035, Australia; [email protected] 3 Department of Microbiology and Infectious Diseases, Nepean Hospital, NSW Health Pathology, Penrith 2747, Australia * Correspondence: [email protected] Abstract: Tick-borne infectious diseases caused by obligate intracellular bacteria of the genus Rick- ettsia are a growing global problem to human and animal health. Surveillance of these pathogens at the wildlife interface is critical to informing public health strategies to limit their impact. In Australia, reptile-associated ticks such as Bothriocroton hydrosauri are the reservoirs for Rickettsia honei, the causative agent of Flinders Island spotted fever. In an effort to gain further insight into the potential for reptile-associated ticks to act as reservoirs for rickettsial infection, Rickettsia-specific PCR screening was performed on 64 Ambylomma albolimbatum ticks taken from shingleback skinks (Tiliqua rugosa) lo- cated in southern Western Australia. PCR screening revealed 92% positivity for rickettsial DNA. PCR Citation: Tadepalli, M.; Vincent, G.; amplification and sequencing of phylogenetically informative rickettsial genes (ompA, ompB, gltA, Hii, S.F.; Watharow, S.; Graves, S.; Stenos, J. -

Gas Gangrene Infection of the Eyes and Orbits
Br J Ophthalmol: first published as 10.1136/bjo.69.2.143 on 1 February 1985. Downloaded from British Journal of Ophthalmology, 1985, 69, 143-148 Gas gangrene infection of the eyes and orbits GERARD W CROCK,' WILSON J HERIOT,' PATTABIRAMAN JANAKIRAMAN,' AND JOHN M WEINER2 From the 'Department of Ophthalmology, University ofMelbourne, and the2C H Greer Pathology Laboratory, the Royal Victorian Eye and Ear Hospital, East Melbourne, Australia SUMMARY The literature on Clostridium perfringens infections is reviewed up to 1983. An additional case is reported with bilateral clostridial infections of the eye and orbit. One eye followed the classical course of relentless panophthalmitis, amaurosis, and orbital cellulitis ending in enucleation. The second eye contained intracameral mud and gas bubbles that were removed by vitrectomy instrumentation. Subsequent removal of the toxic cataract resulted in a final aided visual acuity of 6/18, N8. This is the third report of a retained globe, and we believe the only known case where the patient was left with useful vision. Clostridium perfringens is a ubiquitous Gram- arms, chest, and abdomen. He was admitted to a positive bacillus found in soil and bowel flora. It is the general hospital, where he was examined under most common of four clostridia species identified in anaesthesia, and his injuries were attended to. copyright. cases of gas gangrene in man.' All species are obligate Ocular findings. The right cornea and anterior anaerobes and are usually saprophytic rather than chamber were intact. There was a scleral laceration pathogenic. Clostridium perfringens is a feared con- over the superonasal area of the pars plana with taminant of limb injuries and may result in death due vitreous prolapse. -

Fournier's Gangrene Caused by Listeria Monocytogenes As
CASE REPORT Fournier’s gangrene caused by Listeria monocytogenes as the primary organism Sayaka Asahata MD1, Yuji Hirai MD PhD1, Yusuke Ainoda MD PhD1, Takahiro Fujita MD1, Yumiko Okada DVM PhD2, Ken Kikuchi MD PhD1 S Asahata, Y Hirai, Y Ainoda, T Fujita, Y Okada, K Kikuchi. Une gangrène de Fournier causée par le Listeria Fournier’s gangrene caused by Listeria monocytogenes as the monocytogenes comme organisme primaire primary organism. Can J Infect Dis Med Microbiol 2015;26(1):44-46. Un homme de 70 ans ayant des antécédents de cancer de la langue s’est présenté avec une gangrène de Fournier causée par un Listeria A 70-year-old man with a history of tongue cancer presented with monocytogenes de sérotype 4b. Le débridement chirurgical a révélé un Fournier’s gangrene caused by Listeria monocytogenes serotype 4b. adénocarcinome rectal non diagnostiqué. Le patient n’avait pas Surgical debridement revealed undiagnosed rectal adenocarcinoma. d’antécédents alimentaires ou de voyage apparents, mais a déclaré The patient did not have an apparent dietary or travel history but consommer des sashimis (poisson cru) tous les jours. reported daily consumption of sashimi (raw fish). L’âge avancé et l’immunodéficience causée par l’adénocarcinome rec- Old age and immunodeficiency due to rectal adenocarcinoma may tal ont peut-être favorisé l’invasion directe du L monocytogenes par la have supported the direct invasion of L monocytogenes from the tumeur. Il s’agit du premier cas déclaré de gangrène de Fournier tumour. The present article describes the first reported case of attribuable au L monocytogenes. Les auteurs proposent d’inclure la con- Fournier’s gangrene caused by L monocytogenes. -

Naeglaria and Brain Infections
Can bacteria shrink tumors? Cancer Therapy: The Microbial Approach n this age of advanced injected live Streptococcus medical science and into cancer patients but after I technology, we still the recipients unfortunately continue to hunt for died from subsequent innovative cancer therapies infections, Coley decided to that prove effective and safe. use heat killed bacteria. He Treatments that successfully made a mixture of two heat- eradicate tumors while at the killed bacterial species, By Alan Barajas same time cause as little Streptococcus pyogenes and damage as possible to normal Serratia marcescens. This Alani Barajas is a Research and tissue are the ultimate goal, concoction was termed Development Technician at Hardy but are also not easy to find. “Coley’s toxins.” Bacteria Diagnostics. She earned her bachelor's degree in Microbiology at were either injected into Cal Poly, San Luis Obispo. The use of microorganisms in tumors or into the cancer therapy is not a new bloodstream. During her studies at Cal Poly, much idea but it is currently a of her time was spent as part of the undergraduate research team for the buzzing topic in cancer Cal Poly Dairy Products Technology therapy research. Center studying spore-forming bacteria in dairy products. In the late 1800s, German Currently she is working on new physicians W. Busch and F. chromogenic media formulations for Fehleisen both individually Hardy Diagnostics, both in the observed that certain cancers prepared and powdered forms. began to regress when patients acquired accidental erysipelas (cellulitis) caused by Streptococcus pyogenes. William Coley was the first to use New York surgeon William bacterial injections to treat cancer www.HardyDiagnostics.com patients. -

Kellie ID Emergencies.Pptx
4/24/11 ID Alert! recognizing rapidly fatal infections Susan M. Kellie, MD, MPH Professor of Medicine Division of Infectious Diseases, UNMSOM Hospital Epidemiologist UNMHSC and NMVAHCS Fever and…. Rash and altered mental status Rash Muscle pain Lymphadenopathy Hypotension Shortness of breath Recent travel Abdominal pain and diarrhea Case 1. The cross-country trucker A 30 year-old trucker driving from Oklahoma to California is hospitalized in Deming with fever and headache He is treated with broad-spectrum antibiotics, but deteriorates with obtundation, low platelet count, and a centrifugal petechial rash and is transferred to UNMH 1 4/24/11 What is your diagnosis? What is the differential diagnosis of fever and headache with petechial rash? (in the US) Tickborne rickettsioses ◦ RMSF Bacteria ◦ Neisseria meningitidis Key diagnosis in this case: “doxycycline deficiency” Key vector-borne rickettsioses treated with doxycycline: RMSF-case-fatality 5-10% ◦ Fever, nausea, vomiting, myalgia, anorexia and headache ◦ Maculopapular rash progresses to petechial after 2-4 days of fever ◦ Occasionally without rash Human granulocytotropic anaplasmosis (HGA): case-fatality<1% Human monocytotropic ehrlichiosis (HME): case fatality 2-3% 2 4/24/11 Lab clues in rickettsioses The total white blood cell (WBC) count is typicallynormal in patients with RMSF, but increased numbers of immature bands are generally observed. Thrombocytopenia, mild elevations in hepatic transaminases, and hyponatremia might be observed with RMSF whereas leukopenia -

WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/05 (2006.01) A61P 31/02 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/CA20 14/000 174 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 4 March 2014 (04.03.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 13/790,91 1 8 March 2013 (08.03.2013) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: LABORATOIRE M2 [CA/CA]; 4005-A, rue kind of regional protection available): ARIPO (BW, GH, de la Garlock, Sherbrooke, Quebec J1L 1W9 (CA). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (72) Inventors: LEMIRE, Gaetan; 6505, rue de la fougere, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Sherbrooke, Quebec JIN 3W3 (CA). -

2012 Case Definitions Infectious Disease
Arizona Department of Health Services Case Definitions for Reportable Communicable Morbidities 2012 TABLE OF CONTENTS Definition of Terms Used in Case Classification .......................................................................................................... 6 Definition of Bi-national Case ............................................................................................................................................. 7 ------------------------------------------------------------------------------------------------------- ............................................... 7 AMEBIASIS ............................................................................................................................................................................. 8 ANTHRAX (β) ......................................................................................................................................................................... 9 ASEPTIC MENINGITIS (viral) ......................................................................................................................................... 11 BASIDIOBOLOMYCOSIS ................................................................................................................................................. 12 BOTULISM, FOODBORNE (β) ....................................................................................................................................... 13 BOTULISM, INFANT (β) ................................................................................................................................................... -

Ehrlichiosis and Anaplasmosis Are Tick-Borne Diseases Caused by Obligate Anaplasmosis: Intracellular Bacteria in the Genera Ehrlichia and Anaplasma
Ehrlichiosis and Importance Ehrlichiosis and anaplasmosis are tick-borne diseases caused by obligate Anaplasmosis: intracellular bacteria in the genera Ehrlichia and Anaplasma. These organisms are widespread in nature; the reservoir hosts include numerous wild animals, as well as Zoonotic Species some domesticated species. For many years, Ehrlichia and Anaplasma species have been known to cause illness in pets and livestock. The consequences of exposure vary Canine Monocytic Ehrlichiosis, from asymptomatic infections to severe, potentially fatal illness. Some organisms Canine Hemorrhagic Fever, have also been recognized as human pathogens since the 1980s and 1990s. Tropical Canine Pancytopenia, Etiology Tracker Dog Disease, Ehrlichiosis and anaplasmosis are caused by members of the genera Ehrlichia Canine Tick Typhus, and Anaplasma, respectively. Both genera contain small, pleomorphic, Gram negative, Nairobi Bleeding Disorder, obligate intracellular organisms, and belong to the family Anaplasmataceae, order Canine Granulocytic Ehrlichiosis, Rickettsiales. They are classified as α-proteobacteria. A number of Ehrlichia and Canine Granulocytic Anaplasmosis, Anaplasma species affect animals. A limited number of these organisms have also Equine Granulocytic Ehrlichiosis, been identified in people. Equine Granulocytic Anaplasmosis, Recent changes in taxonomy can make the nomenclature of the Anaplasmataceae Tick-borne Fever, and their diseases somewhat confusing. At one time, ehrlichiosis was a group of Pasture Fever, diseases caused by organisms that mostly replicated in membrane-bound cytoplasmic Human Monocytic Ehrlichiosis, vacuoles of leukocytes, and belonged to the genus Ehrlichia, tribe Ehrlichieae and Human Granulocytic Anaplasmosis, family Rickettsiaceae. The names of the diseases were often based on the host Human Granulocytic Ehrlichiosis, species, together with type of leukocyte most often infected. -

Rhinoscleroma in an Immigrant from Egypt: a Case Report
387 BRIEF COMMUNICATION Rhinoscleroma in an Immigrant From Egypt: A Case Report Edgardo Bonacina, MD,∗ Leonardo Chianura, MD, DTM&H,† Maurizio Sberna, MD,‡ Giuseppe Ortisi, MD,§ Giovanna Gelosa, MD,|| Alberto Citterio, MD,‡ Giovanni Gesu, MD,§ and Massimo Puoti, MD† Downloaded from https://academic.oup.com/jtm/article/19/6/387/1795562 by guest on 23 September 2021 Departments of ∗Pathological Anatomy; †Infectious Diseases; ‡Neuroradiology; §Microbiology, and; ||Otorinolaringoiatry, Niguarda Ca` Granda Hospital, Milano, Italy DOI: 10.1111/j.1708-8305.2012.00659.x Rhinoscleroma is a chronic indolent granulomatous infection of the nose and the upper respiratory tract caused by Klebsiella rhinoscleromatis; this condition is endemic to many regions of the world including North Africa. We present a case of rhinoscleroma in a 51-year-old Egyptian immigrant with 1-month history of epistaxis. We would postulate that with increased travel from areas where rhinoscleroma is endemic to other non-endemic areas, diagnosis of this condition will become more common. hough rarely observed, rhinoscleroma has to be nasal fossae and ethmoid sinuses with complete bony T taken into consideration in travelers returning destruction of bilateral nasal turbinates (Figure 1). with ear, nose, and throat presentations, particularly Endoscopic biopsy was performed under local anesthe- after traveling to developing countries or regions where sia. Histopathologic examination revealed numerous this condition is endemic.1,2 foamy macrophages (Mikulicz cells) containing bacteria (Figure 2); no fungal hyphae were found.3 Staphylococcus Case Report aureus and Klebsiella rhinoscleromatis were isolated by culture of the tissue biopsy. A diagnosis of rhinoscle- A 51-year-old Egyptian male immigrant presented on roma was made. -

Skin and Soft Tissue Infections Ohsuerin Bonura, MD, MCR Oregon Health & Science University Objectives
Difficult Skin and Soft tissue Infections OHSUErin Bonura, MD, MCR Oregon Health & Science University Objectives • Compare and contrast the epidemiology and clinical presentation of common skin and soft tissue diseases • State the management for skin and soft tissue infections OHSU• Differentiate true infection from infectious disease mimics of the skin Casey Casey is a 2 year old boy who presents with this rash. What is the best treatment? A. Soap and Water B. Ibuprofen, it will self OHSUresolve C. Dicloxacillin D. Mupirocin OHSUImpetigo Impetigo Epidemiology and Treatment OHSU Ellen Ellen is a 54 year old morbidly obese woman with DM, HTN and venous stasis who presented with a painful left leg and fever. She has had 3 episodes in the last 6 months. What do you recommend? A. Cefazolin followed by oral amoxicillin prophylaxis B. Vancomycin – this is likely OHSUMRSA C. Amoxicillin – this is likely erysipelas D. Clindamycin to cover staph and strep cellulitis Impetigo OHSUErysipelas Erysipelas Risk: lymphedema, stasis, obesity, paresis, DM, ETOH OHSURecurrence rate: 30% in 3 yrs Treatment: Penicillin Impetigo Erysipelas OHSUCellulitis Cellulitis • DEEPER than erysipelas • Microbiology: – 6-48hrs post op: think GAS… too early for staph (days in the making)! – Periorbital – Staph, Strep pneumoniae, GAS OHSU– Post Varicella - GAS – Skin popping – Staph + almost anything! Framework for Skin and Soft Tissue Infections (SSTIs) NONPurulent Purulent Necrotizing/Cellulitis/Erysipelas Furuncle/Carbuncle/Abscess Severe Moderate Mild Severe Moderate Mild I&D I&D I&D I&D IV Rx Oral Rx C&S C&S C&S C&S Vanc + Pip-tazo OHSUEmpiric IV Empiric MRSA Oral MRSA TMP/SMX Doxy What Are Your “Go-To” Oral Options For Non-Purulent SSTI? Amoxicillin Doxycycline OHSUCephalexin Doxycycline Trimethoprim-Sulfamethoxazole OHSU Miller LG, et al.