Redalyc.Detection of Antibodies to Anaplasma, Bartonella and Coxiella
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distribution of Tick-Borne Diseases in China Xian-Bo Wu1, Ren-Hua Na2, Shan-Shan Wei2, Jin-Song Zhu3 and Hong-Juan Peng2*
Wu et al. Parasites & Vectors 2013, 6:119 http://www.parasitesandvectors.com/content/6/1/119 REVIEW Open Access Distribution of tick-borne diseases in China Xian-Bo Wu1, Ren-Hua Na2, Shan-Shan Wei2, Jin-Song Zhu3 and Hong-Juan Peng2* Abstract As an important contributor to vector-borne diseases in China, in recent years, tick-borne diseases have attracted much attention because of their increasing incidence and consequent significant harm to livestock and human health. The most commonly observed human tick-borne diseases in China include Lyme borreliosis (known as Lyme disease in China), tick-borne encephalitis (known as Forest encephalitis in China), Crimean-Congo hemorrhagic fever (known as Xinjiang hemorrhagic fever in China), Q-fever, tularemia and North-Asia tick-borne spotted fever. In recent years, some emerging tick-borne diseases, such as human monocytic ehrlichiosis, human granulocytic anaplasmosis, and a novel bunyavirus infection, have been reported frequently in China. Other tick-borne diseases that are not as frequently reported in China include Colorado fever, oriental spotted fever and piroplasmosis. Detailed information regarding the history, characteristics, and current epidemic status of these human tick-borne diseases in China will be reviewed in this paper. It is clear that greater efforts in government management and research are required for the prevention, control, diagnosis, and treatment of tick-borne diseases, as well as for the control of ticks, in order to decrease the tick-borne disease burden in China. Keywords: Ticks, Tick-borne diseases, Epidemic, China Review (Table 1) [2,4]. Continuous reports of emerging tick-borne Ticks can carry and transmit viruses, bacteria, rickettsia, disease cases in Shandong, Henan, Hebei, Anhui, and spirochetes, protozoans, Chlamydia, Mycoplasma,Bartonia other provinces demonstrate the rise of these diseases bodies, and nematodes [1,2]. -

Parinaud's Oculoglandular Syndrome
Tropical Medicine and Infectious Disease Case Report Parinaud’s Oculoglandular Syndrome: A Case in an Adult with Flea-Borne Typhus and a Review M. Kevin Dixon 1, Christopher L. Dayton 2 and Gregory M. Anstead 3,4,* 1 Baylor Scott & White Clinic, 800 Scott & White Drive, College Station, TX 77845, USA; [email protected] 2 Division of Critical Care, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA; [email protected] 3 Medical Service, South Texas Veterans Health Care System, San Antonio, TX 78229, USA 4 Division of Infectious Diseases, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA * Correspondence: [email protected]; Tel.: +1-210-567-4666; Fax: +1-210-567-4670 Received: 7 June 2020; Accepted: 24 July 2020; Published: 29 July 2020 Abstract: Parinaud’s oculoglandular syndrome (POGS) is defined as unilateral granulomatous conjunctivitis and facial lymphadenopathy. The aims of the current study are to describe a case of POGS with uveitis due to flea-borne typhus (FBT) and to present a diagnostic and therapeutic approach to POGS. The patient, a 38-year old man, presented with persistent unilateral eye pain, fever, rash, preauricular and submandibular lymphadenopathy, and laboratory findings of FBT: hyponatremia, elevated transaminase and lactate dehydrogenase levels, thrombocytopenia, and hypoalbuminemia. His condition rapidly improved after starting doxycycline. Soon after hospitalization, he was diagnosed with uveitis, which responded to topical prednisolone. To derive a diagnostic and empiric therapeutic approach to POGS, we reviewed the cases of POGS from its various causes since 1976 to discern epidemiologic clues and determine successful diagnostic techniques and therapies; we found multiple cases due to cat scratch disease (CSD; due to Bartonella henselae) (twelve), tularemia (ten), sporotrichosis (three), Rickettsia conorii (three), R. -
If Needed You Can Use Two Lines
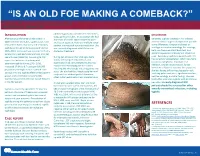
“IS AN OLD FOE MAKING A COMEBACK?” •Eyob Tadesse MD1; Samie Meskele MD1; Ankoor Biswas MD1 •Aurora Health Care, Milwaukee WI. NTRODUCTION abdomen gradually spread to her extremities, DISCUSSION I scalp, palms and soles. In association she had After being on the verge of elimination in shortness of breath, vague abdominal pain Generally, syphilis presents in HIV infected 2000 in the United States, syphilis cases have and loss of appetite, history of multiple sexual patients similar to general population yet with rebounded. Rates of primary and secondary partner, unprotected sex and prostitution. She some differences. Diagnosis is based on syphilis continued to increase overall during was recently diagnosed with HIV but not serologic test and microbiology. For serology, 2005–2013. Increases have occurred primarily started on treatment. both non treponemal antibody test, and among men, and particularly among men has specific treponemal antibody test should be used. Secondary syphilis in patients with HIV sex with men (MSM)(1). According to CDC During her admission her vital signs were has varied skin presentation, which can mimic report the incidence of primary and stable, she had pale conjunctivae, skin cutaneous lymphoma, mycobacterial secondary syphilis during 2015–2016, examination had demonstrated widespread macular and maculopapular skin lesions infection, bacillary angiomatosis, fungal increased 17.6% to 8.7 cases per 100,000 infections or Kaposi’s sarcoma. In our patient, population, the highest rate reported since involving the whole body including palms and soles. She also had thin, fragile scalp hair and she was having diffuse maculopapular rash, 1993(2). HIV and syphilis affect similar patient scalp hair loss without genital ulceration; involving palms and soles, significant hair loss, groups and co-infection is common(3). -

Q Fever in Small Ruminants and Its Public Health Importance
Journal of Dairy & Veterinary Sciences ISSN: 2573-2196 Review Article Dairy and Vet Sci J Volume 9 Issue 1 - January 2019 Copyright © All rights are reserved by Tolera Tagesu Tucho DOI: 10.19080/JDVS.2019.09.555752 Q Fever in Small Ruminants and its Public Health Importance Tolera Tagesu* School of Veterinary Medicine, Jimma University, Ethiopia Submission: December 01, 2018; Published: January 11, 2019 *Corresponding author: Tolera Tagesu Tucho, School of Veterinary Medicine, Jimma University, Jimma Oromia, Ethiopia Abstract Query fever is caused by Coxiella burnetii, it’s a worldwide zoonotic infectious disease where domestic small ruminants are the main reservoirs for human infections. Coxiella burnetii, is a Gram-negative obligate intracellular bacterium, adapted to thrive within the phagolysosome of the phagocyte. Humans become infected primarily by inhaling aerosols that are contaminated with C. burnetii. Ingestion (particularly drinking raw milk) and person-to-person transmission are minor routes. Animals shed the bacterium in urine and feces, and in very high concentrations in birth by-products. The bacterium persists in the environment in a resistant spore-like form which may become airborne and transported long distances by the wind. It is considered primarily as occupational disease of workers in close contact with farm animals or processing their be commenced immediately whenever Q fever is suspected. To prevent both the introduction and spread of Q fever infection, preventive measures shouldproducts, be however,implemented it may including occur also immunization in persons without with currently direct contact. available Doxycycline vaccines drugof domestic is the first small line ruminant of treatment animals for Q and fever. -

Genetic Diversity of Bartonella Species in Small Mammals in the Qaidam
www.nature.com/scientificreports OPEN Genetic diversity of Bartonella species in small mammals in the Qaidam Basin, western China Huaxiang Rao1, Shoujiang Li3, Liang Lu4, Rong Wang3, Xiuping Song4, Kai Sun5, Yan Shi3, Dongmei Li4* & Juan Yu2* Investigation of the prevalence and diversity of Bartonella infections in small mammals in the Qaidam Basin, western China, could provide a scientifc basis for the control and prevention of Bartonella infections in humans. Accordingly, in this study, small mammals were captured using snap traps in Wulan County and Ge’ermu City, Qaidam Basin, China. Spleen and brain tissues were collected and cultured to isolate Bartonella strains. The suspected positive colonies were detected with polymerase chain reaction amplifcation and sequencing of gltA, ftsZ, RNA polymerase beta subunit (rpoB) and ribC genes. Among 101 small mammals, 39 were positive for Bartonella, with the infection rate of 38.61%. The infection rate in diferent tissues (spleens and brains) (χ2 = 0.112, P = 0.738) and gender (χ2 = 1.927, P = 0.165) of small mammals did not have statistical diference, but that in diferent habitats had statistical diference (χ2 = 10.361, P = 0.016). Through genetic evolution analysis, 40 Bartonella strains were identifed (two diferent Bartonella species were detected in one small mammal), including B. grahamii (30), B. jaculi (3), B. krasnovii (3) and Candidatus B. gerbillinarum (4), which showed rodent-specifc characteristics. B. grahamii was the dominant epidemic strain (accounted for 75.0%). Furthermore, phylogenetic analysis showed that B. grahamii in the Qaidam Basin, might be close to the strains isolated from Japan and China. -

HIV and the SKIN • Sudden Acute Exacerbations • Treatment Failure DR
2018/08/13 KEY FEATURES • Atypical presentation of common disorders • Severe or exaggerated presentations HIV AND THE SKIN • Sudden acute exacerbations • Treatment failure DR. FREDAH MALEKA DERMATOLOGY UNIVERSITY OF PRETORIA:KALAFONG VIRAL INFECTIONS EXANTHEM OF PRIMARY HIV INFECTION • Exanthem of primary HIV infection • Acute retroviral syndrome • Herpes simplex virus (HSV) • Morbilliform rash (exanthem) : 2-4 weeks after HIV exposure • Varicella Zoster virus (VZV) • Typically generalised • Molluscum contagiosum (Poxvirus) • Pronounced on face and trunk, sparing distal extremities • Human papillomavirus (HPV) • Associated : fever, lymphadenopathy, pharyngitis • Epstein Barr virus (EBV) • DDX: drug reaction • Cytomegalovirus (CMV) • other viral infections – EBV, Enteroviruses, Hepatitis B virus 1 2018/08/13 HERPES SIMPLEX VIRUS(HSV) • Vesicular eruption due to HSV 1&2 • Primary lesion: painful, grouped vesicles on an erythematous base • HIV: attacks are more frequent and severe • : chronic, non-healing, deep ulcers, with scarring and tissue destruction • CLUE: severe pain and recurrences • DDX: syphilis, chancroid, lymphogranuloma venereum • Tzanck smear, Histology, Viral culture HSV • Treatment: Acyclovir 400mg tds 7-10 days • Alternatives: Valacyclovir and Famciclovir • In setting of treatment failure, viral isolates tested for resistance against acyclovir • Alternative drugs: Foscarnet, Cidofovir • Chronic suppressive therapy ( >8 attacks per year) 2 2018/08/13 VARICELLA • Chickenpox • Presents with erythematous papules and umbilicated -

Coxiella Burnetii
SENTINEL LEVEL CLINICAL LABORATORY GUIDELINES FOR SUSPECTED AGENTS OF BIOTERRORISM AND EMERGING INFECTIOUS DISEASES Coxiella burnetii American Society for Microbiology (ASM) Revised March 2016 For latest revision, see web site below: https://www.asm.org/Articles/Policy/Laboratory-Response-Network-LRN-Sentinel-Level-C ASM Subject Matter Expert: David Welch, Ph.D. Medical Microbiology Consulting Dallas, TX [email protected] ASM Sentinel Laboratory Protocol Working Group APHL Advisory Committee Vickie Baselski, Ph.D. Barbara Robinson-Dunn, Ph.D. Patricia Blevins, MPH University of Tennessee at Department of Clinical San Antonio Metro Health Memphis Pathology District Laboratory Memphis, TN Beaumont Health System [email protected] [email protected] Royal Oak, MI BRobinson- Erin Bowles David Craft, Ph.D. [email protected] Wisconsin State Laboratory of Penn State Milton S. Hershey Hygiene Medical Center Michael A. Saubolle, Ph.D. [email protected] Hershey, PA Banner Health System [email protected] Phoenix, AZ Christopher Chadwick, MS [email protected] Association of Public Health Peter H. Gilligan, Ph.D. m Laboratories University of North Carolina [email protected] Hospitals/ Susan L. Shiflett Clinical Microbiology and Michigan Department of Mary DeMartino, BS, Immunology Labs Community Health MT(ASCP)SM Chapel Hill, NC Lansing, MI State Hygienic Laboratory at the [email protected] [email protected] University of Iowa [email protected] Larry Gray, Ph.D. Alice Weissfeld, Ph.D. TriHealth Laboratories and Microbiology Specialists Inc. Harvey Holmes, PhD University of Cincinnati College Houston, TX Centers for Disease Control and of Medicine [email protected] Prevention Cincinnati, OH om [email protected] [email protected] David Welch, Ph.D. -

WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/05 (2006.01) A61P 31/02 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/CA20 14/000 174 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 4 March 2014 (04.03.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 13/790,91 1 8 March 2013 (08.03.2013) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: LABORATOIRE M2 [CA/CA]; 4005-A, rue kind of regional protection available): ARIPO (BW, GH, de la Garlock, Sherbrooke, Quebec J1L 1W9 (CA). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (72) Inventors: LEMIRE, Gaetan; 6505, rue de la fougere, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Sherbrooke, Quebec JIN 3W3 (CA). -

Rheumatic Manifestations of Bartonella Infection in 2 Children MOHAMMAD J
Case Report Rheumatic Manifestations of Bartonella Infection in 2 Children MOHAMMAD J. AL-MATAR, ROSS E. PETTY, DAVID A. CABRAL, LORI B. TUCKER, BANAFSHI PEYVANDI, JULIE PRENDIVILLE, JACK FORBES, ROBYN CAIRNS, and RALPH ROTHSTEIN ABSTRACT. We describe 2 patients with very unusual rheumatological presentations presumably caused by Bartonella infection: one had myositis of proximal thigh muscles bilaterally, and the other had arthritis and skin nodules. Both patients had very high levels of antibody to Bartonella that decreased in asso- ciation with clinical improvement. Bartonella infection should be considered in the differential diag- nosis of unusual myositis or arthritis in children. (J Rheumatol 2002;29:184–6) Key Indexing Terms: MYOSITIS ARTHRITIS BARTONELLA Infection with Bartonella species has a wide range of mani- was slightly increased at 9.86 IU/l (normal 4.51–9.16), and IgA was 2.1 IU/l festations in children including cat scratch disease (regional (normal 0.2–1.0). C3 was 0.11 g/l (normal 0.77–1.43) and C4 was 0.28 (nor- mal 0.07–0.40). Antinuclear antibodies were present at a titer of 1:40, the anti- granulomatous lymphadenitis), bacillary angiomatosis, streptolysin O titer was 35 (normal < 200), and the anti-DNAase B titer was encephalitis, Parinaud’s oculoglandular syndrome, Trench 1:85 (normal). Urinalysis showed 50–100 erythrocytes and 5–10 leukocytes fever (Vincent’s angina), osteomyelitis, granulomatous per high power field. Routine cultures of urine, blood, and throat were nega- hepatitis, splenitis, pneumonitis, endocarditis, and fever of tive. Liver enzymes, electrolytes, HIV serology, cerebrospinal fluid analysis, unknown origin1-3. -

CD Alert Monthly Newsletter of National Centre for Disease Control, Directorate General of Health Services, Government of India
CD Alert Monthly Newsletter of National Centre for Disease Control, Directorate General of Health Services, Government of India May - July 2009 Vol. 13 : No. 1 SCRUB TYPHUS & OTHER RICKETTSIOSES it lacks lipopolysaccharide and peptidoglycan RICKETTSIAL DISEASES and does not have an outer slime layer. It is These are the diseases caused by rickettsiae endowed with a major surface protein (56kDa) which are small, gram negative bacilli adapted and some minor surface protein (110, 80, 46, to obligate intracellular parasitism, and 43, 39, 35, 25 and 25kDa). There are transmitted by arthropod vectors. These considerable differences in virulence and organisms are primarily parasites of arthropods antigen composition among individual strains such as lice, fleas, ticks and mites, in which of O.tsutsugamushi. O.tsutsugamushi has they are found in the alimentary canal. In many serotypes (Karp, Gillian, Kato and vertebrates, including humans, they infect the Kawazaki). vascular endothelium and reticuloendothelial GLOBAL SCENARIO cells. Commonly known rickettsial disease is Scrub Typhus. Geographic distribution of the disease occurs within an area of about 13 million km2 including- The family Rickettsiaeceae currently comprises Afghanistan and Pakistan to the west; Russia of three genera – Rickettsia, Orientia and to the north; Korea and Japan to the northeast; Ehrlichia which appear to have descended Indonesia, Papua New Guinea, and northern from a common ancestor. Former members Australia to the south; and some smaller of the family, Coxiella burnetii, which causes islands in the western Pacific. It was Q fever and Rochalimaea quintana causing first observed in Japan where it was found to trench fever have been excluded because the be transmitted by mites. -

CASE REPORT the PATIENT 33-Year-Old Woman
CASE REPORT THE PATIENT 33-year-old woman SIGNS & SYMPTOMS – 6-day history of fever Katherine Lazet, DO; – Groin pain and swelling Stephanie Rutterbush, MD – Recent hiking trip in St. Vincent Ascension Colorado Health, Evansville, Ind (Dr. Lazet); Munson Healthcare Ostego Memorial Hospital, Lewiston, Mich (Dr. Rutterbush) [email protected] The authors reported no potential conflict of interest THE CASE relevant to this article. A 33-year-old Caucasian woman presented to the emergency department with a 6-day his- tory of fever (103°-104°F) and right groin pain and swelling. Associated symptoms included headache, diarrhea, malaise, weakness, nausea, cough, and anorexia. Upon presentation, she admitted to a recent hike on a bubonic plague–endemic trail in Colorado. Her vital signs were unremarkable, and the physical examination demonstrated normal findings except for tender, erythematous, nonfluctuant right inguinal lymphadenopathy. The patient was admitted for intractable pain and fever and started on intravenous cefoxitin 2 g IV every 8 hours and oral doxycycline 100 mg every 12 hours for pelvic inflammatory disease vs tick- or flea-borne illness. Due to the patient’s recent trip to a plague-infested area, our suspicion for Yersinia pestis infection was high. The patient’s work-up included a nega- tive pregnancy test and urinalysis. A com- FIGURE 1 plete blood count demonstrated a white CT scan from admission blood cell count of 8.6 (4.3-10.5) × 103/UL was revealing with a 3+ left shift and a platelet count of 112 (180-500) × 103/UL. A complete metabolic panel showed hypokalemia and hyponatremia (potassium 2.8 [3.5-5.1] mmol/L and sodium 134 [137-145] mmol/L). -

The Difference in Clinical Characteristics Between Acute Q Fever and Scrub Typhus in Southern Taiwan
International Journal of Infectious Diseases (2009) 13, 387—393 http://intl.elsevierhealth.com/journals/ijid The difference in clinical characteristics between acute Q fever and scrub typhus in southern Taiwan Chung-Hsu Lai a,b, Chun-Kai Huang a, Hui-Ching Weng c, Hsing-Chun Chung a, Shiou-Haur Liang a, Jiun-Nong Lin a,b, Chih-Wen Lin d, Chuan-Yuan Hsu d, Hsi-Hsun Lin a,* a Division of Infectious Diseases, Department of Internal Medicine, E-Da Hospital/I-Shou University, 1 E-Da Road, Jiau-Shu Tsuen, Yan-Chau Shiang, Kaohsiung County, 824 Taiwan, Republic of China b Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung County, Taiwan, Republic of China c Department of Health Management, I-Shou University, Kaohsiung County, Taiwan, Republic of China d Section of Gastroenterology, Department of Internal Medicine, E-Da Hospital/I-Shou University, Kaohsiung County, Taiwan, Republic of China Received 14 April 2008; received in revised form 17 July 2008; accepted 29 July 2008 Corresponding Editor: Craig Lee, Ottawa, Canada KEYWORDS Summary Acute Q fever; Objective: To identify the differences in clinical characteristics between acute Q fever and scrub Coxiella burnetii; typhus in southern Taiwan. Scrub typhus; Methods: A prospective observational study was conducted in which serological tests for acute Q Orientia tsutsugamushi; fever and scrub typhus were performed simultaneously regardless of which disease was suspected Clinical characteristics; clinically. From April 2004 to December 2007, 80 and 40 cases of serologically confirmed acute Q Taiwan fever and scrub typhus, respectively, were identified and included in the study for comparison.