Growth of Bartonella Henselae in a Biofilm
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Parinaud's Oculoglandular Syndrome
Tropical Medicine and Infectious Disease Case Report Parinaud’s Oculoglandular Syndrome: A Case in an Adult with Flea-Borne Typhus and a Review M. Kevin Dixon 1, Christopher L. Dayton 2 and Gregory M. Anstead 3,4,* 1 Baylor Scott & White Clinic, 800 Scott & White Drive, College Station, TX 77845, USA; [email protected] 2 Division of Critical Care, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA; [email protected] 3 Medical Service, South Texas Veterans Health Care System, San Antonio, TX 78229, USA 4 Division of Infectious Diseases, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA * Correspondence: [email protected]; Tel.: +1-210-567-4666; Fax: +1-210-567-4670 Received: 7 June 2020; Accepted: 24 July 2020; Published: 29 July 2020 Abstract: Parinaud’s oculoglandular syndrome (POGS) is defined as unilateral granulomatous conjunctivitis and facial lymphadenopathy. The aims of the current study are to describe a case of POGS with uveitis due to flea-borne typhus (FBT) and to present a diagnostic and therapeutic approach to POGS. The patient, a 38-year old man, presented with persistent unilateral eye pain, fever, rash, preauricular and submandibular lymphadenopathy, and laboratory findings of FBT: hyponatremia, elevated transaminase and lactate dehydrogenase levels, thrombocytopenia, and hypoalbuminemia. His condition rapidly improved after starting doxycycline. Soon after hospitalization, he was diagnosed with uveitis, which responded to topical prednisolone. To derive a diagnostic and empiric therapeutic approach to POGS, we reviewed the cases of POGS from its various causes since 1976 to discern epidemiologic clues and determine successful diagnostic techniques and therapies; we found multiple cases due to cat scratch disease (CSD; due to Bartonella henselae) (twelve), tularemia (ten), sporotrichosis (three), Rickettsia conorii (three), R. -
If Needed You Can Use Two Lines
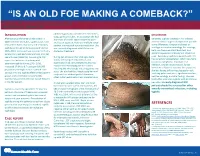
“IS AN OLD FOE MAKING A COMEBACK?” •Eyob Tadesse MD1; Samie Meskele MD1; Ankoor Biswas MD1 •Aurora Health Care, Milwaukee WI. NTRODUCTION abdomen gradually spread to her extremities, DISCUSSION I scalp, palms and soles. In association she had After being on the verge of elimination in shortness of breath, vague abdominal pain Generally, syphilis presents in HIV infected 2000 in the United States, syphilis cases have and loss of appetite, history of multiple sexual patients similar to general population yet with rebounded. Rates of primary and secondary partner, unprotected sex and prostitution. She some differences. Diagnosis is based on syphilis continued to increase overall during was recently diagnosed with HIV but not serologic test and microbiology. For serology, 2005–2013. Increases have occurred primarily started on treatment. both non treponemal antibody test, and among men, and particularly among men has specific treponemal antibody test should be used. Secondary syphilis in patients with HIV sex with men (MSM)(1). According to CDC During her admission her vital signs were has varied skin presentation, which can mimic report the incidence of primary and stable, she had pale conjunctivae, skin cutaneous lymphoma, mycobacterial secondary syphilis during 2015–2016, examination had demonstrated widespread macular and maculopapular skin lesions infection, bacillary angiomatosis, fungal increased 17.6% to 8.7 cases per 100,000 infections or Kaposi’s sarcoma. In our patient, population, the highest rate reported since involving the whole body including palms and soles. She also had thin, fragile scalp hair and she was having diffuse maculopapular rash, 1993(2). HIV and syphilis affect similar patient scalp hair loss without genital ulceration; involving palms and soles, significant hair loss, groups and co-infection is common(3). -

Characterization of the Interaction Between R. Conorii and Human
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 4-5-2018 Characterization of the Interaction Between R. Conorii and Human Host Vitronectin in Rickettsial Pathogenesis Abigail Inez Fish Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Bacteria Commons, Bacteriology Commons, Biology Commons, Immunology of Infectious Disease Commons, and the Pathogenic Microbiology Commons Recommended Citation Fish, Abigail Inez, "Characterization of the Interaction Between R. Conorii and Human Host Vitronectin in Rickettsial Pathogenesis" (2018). LSU Doctoral Dissertations. 4566. https://digitalcommons.lsu.edu/gradschool_dissertations/4566 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. CHARACTERIZATION OF THE INTERACTION BETWEEN R. CONORII AND HUMAN HOST VITRONECTIN IN RICKETTSIAL PATHOGENESIS A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Interdepartmental Program in Biomedical and Veterinary Medical Sciences Through the Department of Pathobiological Sciences by Abigail Inez -

Genetic Diversity of Bartonella Species in Small Mammals in the Qaidam
www.nature.com/scientificreports OPEN Genetic diversity of Bartonella species in small mammals in the Qaidam Basin, western China Huaxiang Rao1, Shoujiang Li3, Liang Lu4, Rong Wang3, Xiuping Song4, Kai Sun5, Yan Shi3, Dongmei Li4* & Juan Yu2* Investigation of the prevalence and diversity of Bartonella infections in small mammals in the Qaidam Basin, western China, could provide a scientifc basis for the control and prevention of Bartonella infections in humans. Accordingly, in this study, small mammals were captured using snap traps in Wulan County and Ge’ermu City, Qaidam Basin, China. Spleen and brain tissues were collected and cultured to isolate Bartonella strains. The suspected positive colonies were detected with polymerase chain reaction amplifcation and sequencing of gltA, ftsZ, RNA polymerase beta subunit (rpoB) and ribC genes. Among 101 small mammals, 39 were positive for Bartonella, with the infection rate of 38.61%. The infection rate in diferent tissues (spleens and brains) (χ2 = 0.112, P = 0.738) and gender (χ2 = 1.927, P = 0.165) of small mammals did not have statistical diference, but that in diferent habitats had statistical diference (χ2 = 10.361, P = 0.016). Through genetic evolution analysis, 40 Bartonella strains were identifed (two diferent Bartonella species were detected in one small mammal), including B. grahamii (30), B. jaculi (3), B. krasnovii (3) and Candidatus B. gerbillinarum (4), which showed rodent-specifc characteristics. B. grahamii was the dominant epidemic strain (accounted for 75.0%). Furthermore, phylogenetic analysis showed that B. grahamii in the Qaidam Basin, might be close to the strains isolated from Japan and China. -

HIV and the SKIN • Sudden Acute Exacerbations • Treatment Failure DR
2018/08/13 KEY FEATURES • Atypical presentation of common disorders • Severe or exaggerated presentations HIV AND THE SKIN • Sudden acute exacerbations • Treatment failure DR. FREDAH MALEKA DERMATOLOGY UNIVERSITY OF PRETORIA:KALAFONG VIRAL INFECTIONS EXANTHEM OF PRIMARY HIV INFECTION • Exanthem of primary HIV infection • Acute retroviral syndrome • Herpes simplex virus (HSV) • Morbilliform rash (exanthem) : 2-4 weeks after HIV exposure • Varicella Zoster virus (VZV) • Typically generalised • Molluscum contagiosum (Poxvirus) • Pronounced on face and trunk, sparing distal extremities • Human papillomavirus (HPV) • Associated : fever, lymphadenopathy, pharyngitis • Epstein Barr virus (EBV) • DDX: drug reaction • Cytomegalovirus (CMV) • other viral infections – EBV, Enteroviruses, Hepatitis B virus 1 2018/08/13 HERPES SIMPLEX VIRUS(HSV) • Vesicular eruption due to HSV 1&2 • Primary lesion: painful, grouped vesicles on an erythematous base • HIV: attacks are more frequent and severe • : chronic, non-healing, deep ulcers, with scarring and tissue destruction • CLUE: severe pain and recurrences • DDX: syphilis, chancroid, lymphogranuloma venereum • Tzanck smear, Histology, Viral culture HSV • Treatment: Acyclovir 400mg tds 7-10 days • Alternatives: Valacyclovir and Famciclovir • In setting of treatment failure, viral isolates tested for resistance against acyclovir • Alternative drugs: Foscarnet, Cidofovir • Chronic suppressive therapy ( >8 attacks per year) 2 2018/08/13 VARICELLA • Chickenpox • Presents with erythematous papules and umbilicated -

Coxiella Burnetii
SENTINEL LEVEL CLINICAL LABORATORY GUIDELINES FOR SUSPECTED AGENTS OF BIOTERRORISM AND EMERGING INFECTIOUS DISEASES Coxiella burnetii American Society for Microbiology (ASM) Revised March 2016 For latest revision, see web site below: https://www.asm.org/Articles/Policy/Laboratory-Response-Network-LRN-Sentinel-Level-C ASM Subject Matter Expert: David Welch, Ph.D. Medical Microbiology Consulting Dallas, TX [email protected] ASM Sentinel Laboratory Protocol Working Group APHL Advisory Committee Vickie Baselski, Ph.D. Barbara Robinson-Dunn, Ph.D. Patricia Blevins, MPH University of Tennessee at Department of Clinical San Antonio Metro Health Memphis Pathology District Laboratory Memphis, TN Beaumont Health System [email protected] [email protected] Royal Oak, MI BRobinson- Erin Bowles David Craft, Ph.D. [email protected] Wisconsin State Laboratory of Penn State Milton S. Hershey Hygiene Medical Center Michael A. Saubolle, Ph.D. [email protected] Hershey, PA Banner Health System [email protected] Phoenix, AZ Christopher Chadwick, MS [email protected] Association of Public Health Peter H. Gilligan, Ph.D. m Laboratories University of North Carolina [email protected] Hospitals/ Susan L. Shiflett Clinical Microbiology and Michigan Department of Mary DeMartino, BS, Immunology Labs Community Health MT(ASCP)SM Chapel Hill, NC Lansing, MI State Hygienic Laboratory at the [email protected] [email protected] University of Iowa [email protected] Larry Gray, Ph.D. Alice Weissfeld, Ph.D. TriHealth Laboratories and Microbiology Specialists Inc. Harvey Holmes, PhD University of Cincinnati College Houston, TX Centers for Disease Control and of Medicine [email protected] Prevention Cincinnati, OH om [email protected] [email protected] David Welch, Ph.D. -

Novel Small Rnas Expressed by Bartonella Bacilliformis Under Multiple Conditions 2 Reveal Potential Mechanisms for Persistence in the Sand Fly Vector and Human 3 Host
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.04.235903; this version posted August 4, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Novel small RNAs expressed by Bartonella bacilliformis under multiple conditions 2 reveal potential mechanisms for persistence in the sand fly vector and human 3 host 4 5 6 Shaun Wachter1, Linda D. Hicks1, Rahul Raghavan2 and Michael F. Minnick1* 7 8 9 10 1 Program in Cellular, Molecular & Microbial Biology, Division of Biological Sciences, 11 University of Montana, Missoula, Montana, United States of America 12 2 Department of Biology and Center for Life in Extreme Environments, Portland State 13 University, Portland, Oregon, United States of America 14 15 16 17 * Corresponding author 18 E-mail: [email protected] (MFM) 19 20 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.04.235903; this version posted August 4, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 21 Abstract 22 Bartonella bacilliformis, the etiological agent of Carrión’s disease, is a Gram-negative, 23 facultative intracellular alphaproteobacterium. Carrión’s disease is an emerging but neglected 24 tropical illness endemic to Peru, Colombia, and Ecuador. B. bacilliformis is spread between 25 humans through the bite of female phlebotomine sand flies. -

“Candidatus Deianiraea Vastatrix” with the Ciliate Paramecium Suggests
bioRxiv preprint doi: https://doi.org/10.1101/479196; this version posted November 27, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. The extracellular association of the bacterium “Candidatus Deianiraea vastatrix” with the ciliate Paramecium suggests an alternative scenario for the evolution of Rickettsiales 5 Castelli M.1, Sabaneyeva E.2, Lanzoni O.3, Lebedeva N.4, Floriano A.M.5, Gaiarsa S.5,6, Benken K.7, Modeo L. 3, Bandi C.1, Potekhin A.8, Sassera D.5*, Petroni G.3* 1. Centro Romeo ed Enrica Invernizzi Ricerca Pediatrica, Dipartimento di Bioscienze, Università 10 degli studi di Milano, Milan, Italy 2. Department of Cytology and Histology, Faculty of Biology, Saint Petersburg State University, Saint-Petersburg, Russia 3. Dipartimento di Biologia, Università di Pisa, Pisa, Italy 4 Centre of Core Facilities “Culture Collections of Microorganisms”, Saint Petersburg State 15 University, Saint Petersburg, Russia 5. Dipartimento di Biologia e Biotecnologie, Università degli studi di Pavia, Pavia, Italy 6. UOC Microbiologia e Virologia, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy 7. Core Facility Center for Microscopy and Microanalysis, Saint Petersburg State University, Saint- Petersburg, Russia 20 8. Department of Microbiology, Faculty of Biology, Saint Petersburg State University, Saint- Petersburg, Russia * Corresponding authors, contacts: [email protected] ; [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/479196; this version posted November 27, 2018. -

09 Piqueras.Qxp
PERSPECTIVES INTERNATIONAL MICROBIOLOGY (2007) 10:217-226 DOI: 10.2436/20.1501.01.30 ISSN: 1139-6709 www.im.microbios.org Microbiology: a dangerous profession? Mercè Piqueras President, Catalan Association for Science Communication (ACCC), Barcelona, Spain The history of science contains many cases of researchers eases in Minorca from the year 1744 to 1749 to which is pre- who have died because of their professional activity. In the fixed, a short account of the climate, productions, inhabi- field of microbiology, some have died or have come close to tants, and endemical distempers of that island (T. Cadell, D. death from infection by agents that were the subject of their Wilson and G. Nicol, London, 1751), which he dedicated to research (Table 1). Infections that had a lethal outcome were the Society of Surgeons of His Majesty’s Royal Navy. usually accidental. Sometimes, however, researchers inocu- Minorcan historian of science Josep M. Vidal Hernández has lated themselves with the pathogen or did not take preventive described and carefully analyzed Cleghorn’s work in measures against the potential pathogen because they wanted Minorca and his report [43]. According to Vidal, what to prove their hypotheses—or disprove someone else’s— Cleghorn describes is “tertian” fever, which was the name regarding the origin of the infection. Here is an overview of given at the time to fever caused by malaria parasites with a several episodes in the history of microbiology since the mid periodicity of 48 hours. In fact, Cleghorn used quinine to nineteenth century involving researchers or workers in fields treat tertian fever (i.e, malaria), which was not eradicated related to microbiology who have become infected. -

WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/05 (2006.01) A61P 31/02 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/CA20 14/000 174 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 4 March 2014 (04.03.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 13/790,91 1 8 March 2013 (08.03.2013) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: LABORATOIRE M2 [CA/CA]; 4005-A, rue kind of regional protection available): ARIPO (BW, GH, de la Garlock, Sherbrooke, Quebec J1L 1W9 (CA). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (72) Inventors: LEMIRE, Gaetan; 6505, rue de la fougere, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Sherbrooke, Quebec JIN 3W3 (CA). -

Fleas and Flea-Borne Diseases
International Journal of Infectious Diseases 14 (2010) e667–e676 Contents lists available at ScienceDirect International Journal of Infectious Diseases journal homepage: www.elsevier.com/locate/ijid Review Fleas and flea-borne diseases Idir Bitam a, Katharina Dittmar b, Philippe Parola a, Michael F. Whiting c, Didier Raoult a,* a Unite´ de Recherche en Maladies Infectieuses Tropicales Emergentes, CNRS-IRD UMR 6236, Faculte´ de Me´decine, Universite´ de la Me´diterrane´e, 27 Bd Jean Moulin, 13385 Marseille Cedex 5, France b Department of Biological Sciences, SUNY at Buffalo, Buffalo, NY, USA c Department of Biology, Brigham Young University, Provo, Utah, USA ARTICLE INFO SUMMARY Article history: Flea-borne infections are emerging or re-emerging throughout the world, and their incidence is on the Received 3 February 2009 rise. Furthermore, their distribution and that of their vectors is shifting and expanding. This publication Received in revised form 2 June 2009 reviews general flea biology and the distribution of the flea-borne diseases of public health importance Accepted 4 November 2009 throughout the world, their principal flea vectors, and the extent of their public health burden. Such an Corresponding Editor: William Cameron, overall review is necessary to understand the importance of this group of infections and the resources Ottawa, Canada that must be allocated to their control by public health authorities to ensure their timely diagnosis and treatment. Keywords: ß 2010 International Society for Infectious Diseases. Published by Elsevier Ltd. All rights reserved. Flea Siphonaptera Plague Yersinia pestis Rickettsia Bartonella Introduction to 16 families and 238 genera have been described, but only a minority is synanthropic, that is they live in close association with The past decades have seen a dramatic change in the geographic humans (Table 1).4,5 and host ranges of many vector-borne pathogens, and their diseases. -

Bartonella Henselae • Fleas and Black-Legged Ticks (Also Called Deer Ticks) of the Genus Ixodes May Serve As Vectors, but This Has Not Disease Agent: Been Proven
APPENDIX 2 Bartonella henselae • Fleas and black-legged ticks (also called deer ticks) of the genus Ixodes may serve as vectors, but this has not Disease Agent: been proven. • Bartonella henselae Blood Phase: Disease Agent Characteristics: • Agent found in endothelial cells and associated with RBCs in symptomatic cases • Gram-negative bacillus or coccobacillus, aerobic, • Occult bacteremia sometimes occurs in the absence nonmotile, nonspore-forming, facultatively intracel- of specific antibodies. lular bacterium • Order: Rhizobiales; Family: Bartonellaceae Survival/Persistence in Blood Products: • Size: 0.3-0.6 ¥ 0.3-1.0 mm • Nucleic acid: Approximately 1900 kb of DNA • A spiking study suggests that B. henselae added to RBCs can be recovered on solid media through 35 Disease Name: days of storage at 4°C. • Cat scratch disease • Cat scratch fever Transmission by Blood Transfusion: • Bacillary angiomatosis • Theoretical • Bacillary peliosis Cases/Frequency in Population: Priority Level: • 22,000 cases per year estimated in the US • Scientific/Epidemiologic evidence regarding blood • 2-6% in US blood donors safety: Theoretical • Cumulative seroprevalence of 7.1% to B. henselae and • Public perception and/or regulatory concern regard- B. quintana in US veterinary professionals ing blood safety: Absent • Public concern regarding disease agent: Very low Incubation Period: Background: • 3-10 days to appearance of papule at inoculation site; regional adenopathy may follow after a few weeks • In 1909,ALBartondescribed organisms that adhered to RBCs. Likelihood of Clinical Disease: • The name Bartonella bacilliformis was used for the • Relatively benign and self-limiting, lasting 6-12 weeks only member of the group identified before 1993. in the absence of antibiotic therapy • Several other species of Bartonella are known to infect humans, but at present, B.