Seroprevalence of Six Pathogens Transmitted by the Ixodes Ricinus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ixodes Ricinus Salivary Serpin Iripin-8 Inhibits the Intrinsic Pathway of Coagulation and Complement
International Journal of Molecular Sciences Article Ixodes ricinus Salivary Serpin Iripin-8 Inhibits the Intrinsic Pathway of Coagulation and Complement Jan Kotál 1,2 , Stéphanie G. I. Polderdijk 3 , Helena Langhansová 1, Monika Ederová 1, Larissa A. Martins 2 , Zuzana Beránková 1, Adéla Chlastáková 1 , OndˇrejHajdušek 4, Michail Kotsyfakis 1,2 , James A. Huntington 3 and JindˇrichChmelaˇr 1,* 1 Department of Medical Biology, Faculty of Science, University of South Bohemia in Ceskˇ é Budˇejovice, Branišovská 1760c, 37005 Ceskˇ é Budˇejovice,Czech Republic; [email protected] (J.K.); [email protected] (H.L.); [email protected] (M.E.); [email protected] (Z.B.); [email protected] (A.C.); [email protected] (M.K.) 2 Laboratory of Genomics and Proteomics of Disease Vectors, Institute of Parasitology, Biology Center CAS, Branišovská 1160/31, 37005 Ceskˇ é Budˇejovice,Czech Republic; [email protected] 3 Cambridge Institute for Medical Research, Department of Haematology, University of Cambridge, The Keith Peters Building, Hills Road, Cambridge CB2 0XY, UK; [email protected] (S.G.I.P.); [email protected] (J.A.H.) 4 Laboratory of Vector Immunology, Institute of Parasitology, Biology Center CAS, Branišovská 1160/31, 37005 Ceskˇ é Budˇejovice,Czech Republic; [email protected] * Correspondence: [email protected] Abstract: Tick saliva is a rich source of antihemostatic, anti-inflammatory, and immunomodulatory molecules that actively help the tick to finish its blood meal. Moreover, these molecules facilitate the Citation: Kotál, J.; Polderdijk, S.G.I.; transmission of tick-borne pathogens. Here we present the functional and structural characterization Langhansová, H.; Ederová, M.; of Iripin-8, a salivary serpin from the tick Ixodes ricinus, a European vector of tick-borne encephalitis Martins, L.A.; Beránková, Z.; and Lyme disease. -

Infectious Disease
INFECTIOUS DISEASE Infectious diseases are caused by germs that are transmitted directly from person to NOTE: person; animal to person (zoonotic The following symbols are used throughout this Community Health Assessment Report to serve only as a simple and quick diseases); from mother to unborn child; or reference for data comparisons and trends for the County. indirectly, such as when a person touches Further analysis may be required before drawing conclusions about the data. a surface that some germs can linger on. The NYSDOH recommends several The apple symbol represents areas in which Oneida County’s status or trend is FAVORABLE or COMPARABLE to its effective strategies for preventing comparison (i.e., NYS, US) or areas/issues identified as infectious diseases,, including: ensuring STRENGTHS. procedures and systems are in place in The magnifying glass symbols represent areas in which Oneida County’s status or trend is UNFAVORABLE to its communities for immunizations to be up to comparison (i.e., NYS, US) or areas/issues of CONCERN date; enabling sanitary practices by or NEED that may warrant further analysis. conveniently located sinks for washing DATA REFERENCES: hands; influencing community resources • All References to tables are in Attachment A – Oneida County Data Book. and cultures to facilitate abstinence and • See also Attachment B – Oneida County Chart Book for risk reduction practices for sexual behavior additional data. and injection drug use, and setting up support systems to ensure medicines are taken as prescribed.453 The reporting of suspect or confirmed communicable diseases is mandated under the New York State Sanitary Code (10NYCRR 2.10). -

Parinaud's Oculoglandular Syndrome
Tropical Medicine and Infectious Disease Case Report Parinaud’s Oculoglandular Syndrome: A Case in an Adult with Flea-Borne Typhus and a Review M. Kevin Dixon 1, Christopher L. Dayton 2 and Gregory M. Anstead 3,4,* 1 Baylor Scott & White Clinic, 800 Scott & White Drive, College Station, TX 77845, USA; [email protected] 2 Division of Critical Care, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA; [email protected] 3 Medical Service, South Texas Veterans Health Care System, San Antonio, TX 78229, USA 4 Division of Infectious Diseases, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA * Correspondence: [email protected]; Tel.: +1-210-567-4666; Fax: +1-210-567-4670 Received: 7 June 2020; Accepted: 24 July 2020; Published: 29 July 2020 Abstract: Parinaud’s oculoglandular syndrome (POGS) is defined as unilateral granulomatous conjunctivitis and facial lymphadenopathy. The aims of the current study are to describe a case of POGS with uveitis due to flea-borne typhus (FBT) and to present a diagnostic and therapeutic approach to POGS. The patient, a 38-year old man, presented with persistent unilateral eye pain, fever, rash, preauricular and submandibular lymphadenopathy, and laboratory findings of FBT: hyponatremia, elevated transaminase and lactate dehydrogenase levels, thrombocytopenia, and hypoalbuminemia. His condition rapidly improved after starting doxycycline. Soon after hospitalization, he was diagnosed with uveitis, which responded to topical prednisolone. To derive a diagnostic and empiric therapeutic approach to POGS, we reviewed the cases of POGS from its various causes since 1976 to discern epidemiologic clues and determine successful diagnostic techniques and therapies; we found multiple cases due to cat scratch disease (CSD; due to Bartonella henselae) (twelve), tularemia (ten), sporotrichosis (three), Rickettsia conorii (three), R. -

Severe Babesiosis Caused by Babesia Divergens in a Host with Intact Spleen, Russia, 2018 T ⁎ Irina V
Ticks and Tick-borne Diseases 10 (2019) 101262 Contents lists available at ScienceDirect Ticks and Tick-borne Diseases journal homepage: www.elsevier.com/locate/ttbdis Severe babesiosis caused by Babesia divergens in a host with intact spleen, Russia, 2018 T ⁎ Irina V. Kukinaa, Olga P. Zelyaa, , Tatiana M. Guzeevaa, Ludmila S. Karanb, Irina A. Perkovskayac, Nina I. Tymoshenkod, Marina V. Guzeevad a Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation b Central Research Institute of Epidemiology, Moscow, Russian Federation c Infectious Clinical Hospital №2 of the Moscow Department of Health, Moscow, Russian Federation d Centre for Hygiene and Epidemiology in Moscow, Moscow, Russian Federation ARTICLE INFO ABSTRACT Keywords: We report a case of severe babesiosis caused by the bovine pathogen Babesia divergens with the development of Protozoan parasites multisystem failure in a splenic host. Immunosuppression other than splenectomy can also predispose people to Babesia divergens B. divergens. There was heavy multiple invasion of up to 14 parasites inside the erythrocyte, which had not been Ixodes ricinus previously observed even in asplenic hosts. The piroplasm 18S rRNA sequence from our patient was identical B. Tick-borne disease divergens EU lineage with identity 99.5–100%. Human babesiosis 1. Introduction Leucocyte left shift with immature neutrophils, signs of dysery- thropoiesis, anisocytosis, and poikilocytosis were seen on the peripheral Babesia divergens, a protozoan blood parasite (Apicomplexa: smear. Numerous intra-erythrocytic parasites were found, which were Babesiidae) is primarily specific to bovines. This parasite is widespread initially falsely identified as Plasmodium falciparum. The patient was throughout Europe within the vector Ixodes ricinus. -
If Needed You Can Use Two Lines
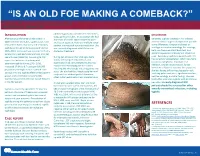
“IS AN OLD FOE MAKING A COMEBACK?” •Eyob Tadesse MD1; Samie Meskele MD1; Ankoor Biswas MD1 •Aurora Health Care, Milwaukee WI. NTRODUCTION abdomen gradually spread to her extremities, DISCUSSION I scalp, palms and soles. In association she had After being on the verge of elimination in shortness of breath, vague abdominal pain Generally, syphilis presents in HIV infected 2000 in the United States, syphilis cases have and loss of appetite, history of multiple sexual patients similar to general population yet with rebounded. Rates of primary and secondary partner, unprotected sex and prostitution. She some differences. Diagnosis is based on syphilis continued to increase overall during was recently diagnosed with HIV but not serologic test and microbiology. For serology, 2005–2013. Increases have occurred primarily started on treatment. both non treponemal antibody test, and among men, and particularly among men has specific treponemal antibody test should be used. Secondary syphilis in patients with HIV sex with men (MSM)(1). According to CDC During her admission her vital signs were has varied skin presentation, which can mimic report the incidence of primary and stable, she had pale conjunctivae, skin cutaneous lymphoma, mycobacterial secondary syphilis during 2015–2016, examination had demonstrated widespread macular and maculopapular skin lesions infection, bacillary angiomatosis, fungal increased 17.6% to 8.7 cases per 100,000 infections or Kaposi’s sarcoma. In our patient, population, the highest rate reported since involving the whole body including palms and soles. She also had thin, fragile scalp hair and she was having diffuse maculopapular rash, 1993(2). HIV and syphilis affect similar patient scalp hair loss without genital ulceration; involving palms and soles, significant hair loss, groups and co-infection is common(3). -

Chlamydia-English
URGENT and PRIVATE IMPORTANT INFORMATION ABOUT YOUR HEALTH DIRECTIONS FOR SEX PARTNERS OF PERSONS WITH CHLAMYDIA PLEASE READ THIS VERY CAREFULLY Your sex partner has recently been treated for chlamydia. Chlamydia is a sexually transmitted disease (STD) that you can get from having any kind of sex (oral, vaginal, or anal) with a person who already has it. You may have been exposed. The good news is that it’s easily treated. You are being given a medicine called azithromycin (sometimes known as “Zithromax”) to treat your chlamydia. Your partner may have given you the actual medicine, or a prescription that you can take to a pharmacy. These are instructions for how to take azithromycin. The best way to take care of this infection is to see your own doctor or clinic provider right away. If you can’t get to a doctor in the next several days, you should take the azithromycin. Even if you decide to take the medicine, it is very important to see a doctor as soon as you can, to get tested for other STDs. People can have more than one STD at the same time. Azithromycin will not cure other sexually transmitted infections. Having STDs can increase your risk of getting HIV, so make sure to also get an HIV test. SYMPTOMS Some people with chlamydia have symptoms, but most do not. Symptoms may include pain in your testicles, pelvis, or lower part of your belly. You may also have pain when you urinate or when having sex. Many people with chlamydia do not know they are infected because they feel fine. -

Genetic Diversity of Bartonella Species in Small Mammals in the Qaidam
www.nature.com/scientificreports OPEN Genetic diversity of Bartonella species in small mammals in the Qaidam Basin, western China Huaxiang Rao1, Shoujiang Li3, Liang Lu4, Rong Wang3, Xiuping Song4, Kai Sun5, Yan Shi3, Dongmei Li4* & Juan Yu2* Investigation of the prevalence and diversity of Bartonella infections in small mammals in the Qaidam Basin, western China, could provide a scientifc basis for the control and prevention of Bartonella infections in humans. Accordingly, in this study, small mammals were captured using snap traps in Wulan County and Ge’ermu City, Qaidam Basin, China. Spleen and brain tissues were collected and cultured to isolate Bartonella strains. The suspected positive colonies were detected with polymerase chain reaction amplifcation and sequencing of gltA, ftsZ, RNA polymerase beta subunit (rpoB) and ribC genes. Among 101 small mammals, 39 were positive for Bartonella, with the infection rate of 38.61%. The infection rate in diferent tissues (spleens and brains) (χ2 = 0.112, P = 0.738) and gender (χ2 = 1.927, P = 0.165) of small mammals did not have statistical diference, but that in diferent habitats had statistical diference (χ2 = 10.361, P = 0.016). Through genetic evolution analysis, 40 Bartonella strains were identifed (two diferent Bartonella species were detected in one small mammal), including B. grahamii (30), B. jaculi (3), B. krasnovii (3) and Candidatus B. gerbillinarum (4), which showed rodent-specifc characteristics. B. grahamii was the dominant epidemic strain (accounted for 75.0%). Furthermore, phylogenetic analysis showed that B. grahamii in the Qaidam Basin, might be close to the strains isolated from Japan and China. -

Borrelia Burgdorferi and Treponema Pallidum: a Comparison of Functional Genomics, Environmental Adaptations, and Pathogenic Mechanisms
PERSPECTIVE SERIES Bacterial polymorphisms Martin J. Blaser and James M. Musser, Series Editors Borrelia burgdorferi and Treponema pallidum: a comparison of functional genomics, environmental adaptations, and pathogenic mechanisms Stephen F. Porcella and Tom G. Schwan Laboratory of Human Bacterial Pathogenesis, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, NIH, Hamilton, Montana, USA Address correspondence to: Tom G. Schwan, Rocky Mountain Laboratories, 903 South 4th Street, Hamilton, Montana 59840, USA. Phone: (406) 363-9250; Fax: (406) 363-9445; E-mail: [email protected]. Spirochetes are a diverse group of bacteria found in (6–8). Here, we compare the biology and genomes of soil, deep in marine sediments, commensal in the gut these two spirochetal pathogens with reference to their of termites and other arthropods, or obligate parasites different host associations and modes of transmission. of vertebrates. Two pathogenic spirochetes that are the focus of this perspective are Borrelia burgdorferi sensu Genomic structure lato, a causative agent of Lyme disease, and Treponema A striking difference between B. burgdorferi and T. pal- pallidum subspecies pallidum, the agent of venereal lidum is their total genomic structure. Although both syphilis. Although these organisms are bound togeth- pathogens have small genomes, compared with many er by ancient ancestry and similar morphology (Figure well known bacteria such as Escherichia coli and Mycobac- 1), as well as by the protean nature of the infections terium tuberculosis, the genomic structure of B. burgdorferi they cause, many differences exist in their life cycles, environmental adaptations, and impact on human health and behavior. The specific mechanisms con- tributing to multisystem disease and persistent, long- term infections caused by both organisms in spite of significant immune responses are not yet understood. -

Pdf/Bookshelf NBK368467.Pdf
BMJ 2019;365:l4159 doi: 10.1136/bmj.l4159 (Published 28 June 2019) Page 1 of 11 Practice BMJ: first published as 10.1136/bmj.l4159 on 28 June 2019. Downloaded from PRACTICE CLINICAL UPDATES Syphilis OPEN ACCESS Patrick O'Byrne associate professor, nurse practitioner 1 2, Paul MacPherson infectious disease specialist 3 1School of Nursing, University of Ottawa, Ottawa, Ontario K1H 8M5, Canada; 2Sexual Health Clinic, Ottawa Public Health, Ottawa, Ontario K1N 5P9; 3Division of Infectious Diseases, Ottawa Hospital General Campus, Ottawa, Ontario What you need to know Box 1: Symptoms of syphilis by stage of infection (see fig 1) • Incidence rates of syphilis have increased substantially around the Primary world, mostly affecting men who have sex with men and people infected • Symptoms appear 10-90 days (mean 21 days) after exposure with HIV http://www.bmj.com/ • Main symptom is a <2 cm chancre: • Have a high index of suspicion for syphilis in any sexually active patient – Progresses from a macule to papule to ulcer over 7 days with genital lesions or rashes – Painless, solitary, indurated, clean base (98% specific, 31% sensitive) • Primary syphilis classically presents as a single, painless, indurated genital ulcer (chancre), but this presentation is only 31% sensitive; – On glans, corona, labia, fourchette, or perineum lesions can be painful, multiple, and extra-genital – A third are extragenital in men who have sex with men and in women • Diagnosis is usually based on serology, using a combination of treponemal and non-treponemal tests. Syphilis remains sensitive to • Localised painless adenopathy benzathine penicillin G Secondary on 24 September 2021 by guest. -

HIV and the SKIN • Sudden Acute Exacerbations • Treatment Failure DR
2018/08/13 KEY FEATURES • Atypical presentation of common disorders • Severe or exaggerated presentations HIV AND THE SKIN • Sudden acute exacerbations • Treatment failure DR. FREDAH MALEKA DERMATOLOGY UNIVERSITY OF PRETORIA:KALAFONG VIRAL INFECTIONS EXANTHEM OF PRIMARY HIV INFECTION • Exanthem of primary HIV infection • Acute retroviral syndrome • Herpes simplex virus (HSV) • Morbilliform rash (exanthem) : 2-4 weeks after HIV exposure • Varicella Zoster virus (VZV) • Typically generalised • Molluscum contagiosum (Poxvirus) • Pronounced on face and trunk, sparing distal extremities • Human papillomavirus (HPV) • Associated : fever, lymphadenopathy, pharyngitis • Epstein Barr virus (EBV) • DDX: drug reaction • Cytomegalovirus (CMV) • other viral infections – EBV, Enteroviruses, Hepatitis B virus 1 2018/08/13 HERPES SIMPLEX VIRUS(HSV) • Vesicular eruption due to HSV 1&2 • Primary lesion: painful, grouped vesicles on an erythematous base • HIV: attacks are more frequent and severe • : chronic, non-healing, deep ulcers, with scarring and tissue destruction • CLUE: severe pain and recurrences • DDX: syphilis, chancroid, lymphogranuloma venereum • Tzanck smear, Histology, Viral culture HSV • Treatment: Acyclovir 400mg tds 7-10 days • Alternatives: Valacyclovir and Famciclovir • In setting of treatment failure, viral isolates tested for resistance against acyclovir • Alternative drugs: Foscarnet, Cidofovir • Chronic suppressive therapy ( >8 attacks per year) 2 2018/08/13 VARICELLA • Chickenpox • Presents with erythematous papules and umbilicated -

Can Leptospirosis Be Treated Without Any Kind of Medication?
ISSN: 2573-9565 Research Article Journal of Clinical Review & Case Reports Can Leptospirosis Be Treated Without Any Kind of Medication? Huang W L* *Corresponding author Huang Wei Ling, Rua Homero Pacheco Alves, 1929, Franca, Sao Paulo, Infectologist, general practitioner, nutrition doctor, acupuncturist, 14400-010, Brazil, Tel: (+55 16) 3721-2437; E-mail: [email protected] pain management, Medical Acupuncture and Pain Management Clinic, Franca, Sao Paulo, Brazil Submitted: 16 Apr 2018; Accepted: 23 Apr 2018; Published: 10 May 2018 Abstract Introduction: Leptospirosis is an acute infectious disease caused by pathogenic Leptospira. Spread in a variety of ways, though the digestive tract infection is the main route of infection. As the disease pathogen final position in the kidney, the urine has an important role in the proliferation of the disease spreading [1]. Purpose: The purpose of this study was to show if leptospirosis can be treated without any kind of medication. The methodology used was the presentation of one case report of a woman presenting three days of generalized pain all over her body, especially in her muscles, mainly the calves of her legs, fever, headache and trembling. A blood exam was asked, as well as serology and acupuncture to relieve her symptoms. Findings: she recovered very well after five sessions of Acupuncture once a day. A month later, she came back with the results of her serology: it was positive leptospirosis. Conclusion: In this case, leptospirosis was cured without the use any kind of medication, being acupuncture a good therapeutic option, reducing the necessity of the patient’s admittance into a hospital, minimizing the costs of the treatmentand restoring the patient to a normal life very quickly. -

WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/05 (2006.01) A61P 31/02 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/CA20 14/000 174 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 4 March 2014 (04.03.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 13/790,91 1 8 March 2013 (08.03.2013) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: LABORATOIRE M2 [CA/CA]; 4005-A, rue kind of regional protection available): ARIPO (BW, GH, de la Garlock, Sherbrooke, Quebec J1L 1W9 (CA). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (72) Inventors: LEMIRE, Gaetan; 6505, rue de la fougere, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Sherbrooke, Quebec JIN 3W3 (CA).