疑似fludiazepam 引起EPS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
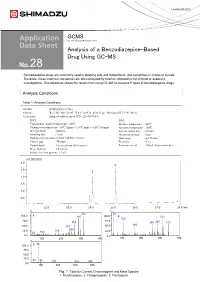
Analysis of a Benzodiazepine-Based Drug Using GC-MS 28
LAAN-E-MS-E028 GCMS Gas Chromatograph Mass Spectrometer Analysis of a Benzodiazepine-Based Drug Using GC-MS 28 Benzodiazepine drugs are commonly used in sleeping aids and tranquilizers, and sometimes in crimes or suicide. Therefore, these chemical substances are often analyzed by forensic laboratories for criminal or academic investigations. This datasheet shows the results from using GC-MS to measure 9 types of benzodiazepine drugs. Analysis Conditions Table 1: Analysis Conditions GC-MS :GCMS-QP2010 Ultra Column : Rxi®-5Sil MS (30 mL. X 0.25 mmI.D., df=0.25 µm, Shimadzu GLC P/N:13623) Glass insert :Silanized splitless insert (P/N: 221-48876-03) [GC] [MS] Vaporization chamber temperature : 260℃ Interface temperature : 280℃ Column oven temperature : 60℃ (2min) -> (10℃/min) -> 320℃ (10min) Ion source temperature : 200℃ Injection mode : Splitless Solvent elution time : 2.0 min Sampling time : 1 min Measurement mode : Scan High pressure injection method: 250 kPa (1.5 min) Mass range : m/z 35-600 Carrier gas : Helium Event time : 0.3 sec Control mode :Linear velocity (45.6 cm/sec) Emission current : 150 µA (high sensitivity) Purge flow rate :3.0 ml/min Sample injection quantity :1.0 µL (x1,000,000) 3.0 3 2.5 2 2.0 1.5 1.0 1 0.5 22.0 23.0 24.0 25.0 26.0 27.0 28.0 min % % 312 55 100.0 1 285 100.0 2 313 109 75.0 75.0 266 259287 342 166 50.0 238 50.0 248 25.0 183 25.0 63 109 0.0 0.0 100 200 300 400 100 200 300 400 % 86 100.0 3 75.0 50.0 25.0 58 99 183 315 387 0.0 100 200 300 400 Fig. -

Recommended Methods for the Identification and Analysis of Fentanyl and Its Analogues in Biological Specimens
Recommended methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2017 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/53 Original language: English © United Nations, November 2017. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. Barry Logan, Center for Forensic Science Research and Education, at the Fredric Rieders Family Founda- tion and NMS Labs, United States; Amanda L.A. -

124.210 Schedule IV — Substances Included. 1
1 CONTROLLED SUBSTANCES, §124.210 124.210 Schedule IV — substances included. 1. Schedule IV shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. 2. Narcotic drugs. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation containing any of the following narcotic drugs, or their salts calculated as the free anhydrous base or alkaloid, in limited quantities as set forth below: a. Not more than one milligram of difenoxin and not less than twenty-five micrograms of atropine sulfate per dosage unit. b. Dextropropoxyphene (alpha-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2- propionoxybutane). c. 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol, its salts, optical and geometric isomers and salts of these isomers (including tramadol). 3. Depressants. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances, including its salts, isomers, and salts of isomers whenever the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation: a. Alprazolam. b. Barbital. c. Bromazepam. d. Camazepam. e. Carisoprodol. f. Chloral betaine. g. Chloral hydrate. h. Chlordiazepoxide. i. Clobazam. j. Clonazepam. k. Clorazepate. l. Clotiazepam. m. Cloxazolam. n. Delorazepam. o. Diazepam. p. Dichloralphenazone. q. Estazolam. r. Ethchlorvynol. s. Ethinamate. t. Ethyl Loflazepate. u. Fludiazepam. v. Flunitrazepam. w. Flurazepam. x. Halazepam. y. Haloxazolam. z. Ketazolam. aa. Loprazolam. ab. Lorazepam. ac. Lormetazepam. ad. Mebutamate. ae. Medazepam. af. Meprobamate. ag. Methohexital. ah. Methylphenobarbital (mephobarbital). -

S1 Table. List of Medications Analyzed in Present Study Drug
S1 Table. List of medications analyzed in present study Drug class Drugs Propofol, ketamine, etomidate, Barbiturate (1) (thiopental) Benzodiazepines (28) (midazolam, lorazepam, clonazepam, diazepam, chlordiazepoxide, oxazepam, potassium Sedatives clorazepate, bromazepam, clobazam, alprazolam, pinazepam, (32 drugs) nordazepam, fludiazepam, ethyl loflazepate, etizolam, clotiazepam, tofisopam, flurazepam, flunitrazepam, estazolam, triazolam, lormetazepam, temazepam, brotizolam, quazepam, loprazolam, zopiclone, zolpidem) Fentanyl, alfentanil, sufentanil, remifentanil, morphine, Opioid analgesics hydromorphone, nicomorphine, oxycodone, tramadol, (10 drugs) pethidine Acetaminophen, Non-steroidal anti-inflammatory drugs (36) (celecoxib, polmacoxib, etoricoxib, nimesulide, aceclofenac, acemetacin, amfenac, cinnoxicam, dexibuprofen, diclofenac, emorfazone, Non-opioid analgesics etodolac, fenoprofen, flufenamic acid, flurbiprofen, ibuprofen, (44 drugs) ketoprofen, ketorolac, lornoxicam, loxoprofen, mefenamiate, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, pranoprofen, proglumetacin, sulindac, talniflumate, tenoxicam, tiaprofenic acid, zaltoprofen, morniflumate, pelubiprofen, indomethacin), Anticonvulsants (7) (gabapentin, pregabalin, lamotrigine, levetiracetam, carbamazepine, valproic acid, lacosamide) Vecuronium, rocuronium bromide, cisatracurium, atracurium, Neuromuscular hexafluronium, pipecuronium bromide, doxacurium chloride, blocking agents fazadinium bromide, mivacurium chloride, (12 drugs) pancuronium, gallamine, succinylcholine -

Report on the Investigation Results
Pharmaceuticals and Medical Devices Agency This English version is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. Report on the Investigation Results February 28, 2017 Pharmaceuticals and Medical Devices Agency I. Overview of Product [Non-proprietary name] See Attachment 1 [Brand name] See Attachment 1 [Approval holder] See Attachment 1 [Indications] See Attachment 1 [Dosage and administration] See Attachment 1 [Investigating office] Office of Safety II 1 Pharmaceuticals and Medical Devices Agency This English version is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. II. Background of the investigation 1. Status in Japan Hypnotics and anxiolytics are prescribed by various specialties and widely used in clinical practice. In particular, benzodiazepine (BZ) receptor agonists, which act on BZ receptors, bind to gamma-aminobutyric acid (GABA)A-BZ receptor complex and enhance the function of GABAA receptors. This promotes neurotransmission of inhibitory systems and demonstrates hypnotic/sedative effects, anxiolytic effects, muscle relaxant effects, and antispasmodic effects. Since the approval of chlordiazepoxide in March 1961, many BZ receptor agonists have been approved as hypnotics and anxiolytics. Currently, hypnotics and anxiolytics are causative agents of drug-related disorders such as drug dependence in Japanese clinical practice. Hypnotics and anxiolitics that rank high in causative agents are BZ receptor agonists for which high frequencies of high doses and multidrug prescriptions have been reported (Japanese Journal of Clinical Psychopharmacology 2013; 16(6): 803-812, Modern Physician 2014; 34(6): 653-656, etc.). -

A Review of the Evidence of Use and Harms of Novel Benzodiazepines
ACMD Advisory Council on the Misuse of Drugs Novel Benzodiazepines A review of the evidence of use and harms of Novel Benzodiazepines April 2020 1 Contents 1. Introduction ................................................................................................................................. 4 2. Legal control of benzodiazepines .......................................................................................... 4 3. Benzodiazepine chemistry and pharmacology .................................................................. 6 4. Benzodiazepine misuse............................................................................................................ 7 Benzodiazepine use with opioids ................................................................................................... 9 Social harms of benzodiazepine use .......................................................................................... 10 Suicide ............................................................................................................................................. 11 5. Prevalence and harm summaries of Novel Benzodiazepines ...................................... 11 1. Flualprazolam ......................................................................................................................... 11 2. Norfludiazepam ....................................................................................................................... 13 3. Flunitrazolam .......................................................................................................................... -

II.3.4 Benzodiazepines by Hiroshi Seno and Hideki Hattori
3.4 II.3.4 Benzodiazepines by Hiroshi Seno and Hideki Hattori Introduction Benzodiazepines show antianxiety, hypnotic, anticonvulsant and muscle-relaxant eff ects. Th is group of drugs has wide safety dose ranges; it means that the ratio of the LD50 to the ED50 (therapeutic index) is high. Because of its safety, benzodiazepines are being widely used in the world. Some of benzodiazepines are also being abused or used for so-called “ drug facilitated sexual assault”, and thus they are under the control of the Narcotics and Psychotropics Control Law; in Japan, triazolam abuse has become one of the serious social problems. In this chapter, a GC/MS method for simultaneous analysis of 22 kinds of benzodiazepines listed in > Table 4.1 is described. In addition, the LC/MS analysis of triazolam, and its metabolites 4-hydroxy- triazolam and α-hydroxytriazolam is also presented. GC/MS analysis of benzodiazepines in blood and urine Reagents and their preparation • Th e pure powder of the 22 kinds of benzodiazepines was donated by each pharmaceutical manufacturers according to the authors’ request a (some of benzodiazepines now obtaina- ble from Sigma, St. Louis, MO, USA). • 1 M Sodium bicarbonate solution: a 8.4-g aliquot of sodium bicarbonate is dissolved in distilled water to prepare 100 mL solution. • 2 M Sodium acetate solution: a 27.5-g aliquot of sodium acetate is dissolved in distilled water to prepare 100 mL solution. GC/MS conditions Column: a DB-5 fused silica capillary column (30 m × 0.25 mm i.d., fi lm thickness 0.25 µm, J & W Scientifi c, Folsom, CA, USA). -

PMDA Alert for Proper Use of Drugs When Using Benzodiazepine
■ PMDA Alert for Proper Use of Drugs https://www.pmda.go.jp/english/safety/info-services/drugs/properly- No. 11 March 2017 use-alert/0001.html PMDA Alert for Proper Use of Drugs Pharmaceuticals and Medical Devices Agency No. 11 March 2017 Dependence associated with Benzodiazepine Receptor Agonists [To Patients] This document is for healthcare professionals. If taking the drug, please consult with your physicians or pharmacists. Please don’t reduce the dosage or stop taking the drug on self-judgment. Benzodiazepine receptor agonists have a characteristic of developing physical dependence with long-term use even within an approved dose range, leading to various withdrawal symptoms on dose reduction or discontinuation. <Major withdrawal symptoms> insomnia, anxiety, feeling irritated, headache, queasy/vomiting, delirium, tremor, seizure, etc. Please pay careful attention to the following when using benzodiazepine receptor agonists as hypnotics-sedatives and anxiolytics. Healthcare professionals should avoid long-term use with chronic administration. - Dependence may occur with long-term use even within an approved dose range. - Therapeutic necessity should be carefully considered when continuing administration of the drug. Healthcare professionals should adhere to the dosage and confirm that there is no multiple prescription of similar drugs. - Long-term administration, high-dose administration, or multiple medications increase the risk of developing dependence. - Healthcare professionals should confirm that similar drugs are not prescribed by other medical institutions. Healthcare professionals should reduce the dose or discontinue carefully such as by gradual dose reduction or alternate-days administration when discontinuing the administration. - Sudden discontinuation will develop serious withdrawal symptoms in addition to aggravate primary diseases. -

Use and Abuse of Benzodiazepines*
A rticles in the Update series Les articles de la rubrique give a concise, authoritative. Le point fournissent un and up-to-date wurvev of the bhilan concis et fiable de la present position in the se- I .ituation actuelle dans le lected fields, and, over a domaine considere. Des ex- ,/t a e period of years, will cover perts couvriront ainsi .vucl- man v different aspe ts of cessivement de nombreux the biomedical sciences aspects des sciences bio- L e p ointt atid public health. .Mo.st oJf mdicales et de la sante the articles will be writ- publique. La plupart de ces ten, by invitation. bv ic- articles auront donc ete A/nowledged experts on the rediges stir demande par les subject. specialistes les plus autorises. Bulletin ofthe World Health Organization, 61 (4): 551 - 562 (1983) © World Health Organization 1983 Use and abuse of benzodiazepines* WHO REVIEW GROUP Benzodiazepines are widely used for the treatment of anxiety, insomnia, and certain neuromuscular and convulsive disorders. However, their widespread avail- ability has given rise to fears that they are over-prescribed. The problem is compounded by the fact that there is no universal agreement among medical prac- titioners as to the clinical indications warranting the use of these drugs. Although most industrialized countries exercise control over the sale and manufacture of benzodiazepines, many developing countries do not have sufficient control of these drugs. As a result, information on drug utilization and associated problems is diffi- cult to obtain and there is a lack ofcomparative data on drug consumption in differ- ent countries. -

Prescription & Illicit Drug Certified Reference Materials
Prescription & Illicit Drug Certified Reference Materials Many analgesics clinically prescribed to manage chronic pain carry warranted concerns over safety and abuse liability. Further complications can arise from drug-drug interactions when stimulants or other illicit compounds are also abused. Cayman Chemical offers Certified Reference Materials (CRMs) to help identify, confirm, and quantify many of the major opioids, benzodiazepines, psychoactive drugs, and other controlled substances. Our CRMs meet ISO/IEC 17025 and ISO 17034 standards and are sold as quantitative solutions in sealed ampules. Opioid CRMs Benzodiazepine CRMs Item No. Product Name Item No. Product Name ISO60128 Acetyl fentanyl (hydrochloride) (CRM) ISO60185 Alprazolam (CRM) 23060 Acrylfentanyl (hydrochloride) (CRM) ISO60181 Clonazepam (CRM) 25936 Benzyl fentanyl (hydrochloride) (CRM) 25614 Clonazolam (CRM) ISO60178 Buprenorphine (hydrochloride) (CRM) ISO60177 Diazepam (CRM) 19734 Butyryl fentanyl (hydrochloride) (CRM) 28623 Diclazepam (CRM) 19884 Carfentanil (CRM) 20991 Etizolam (CRM) 26637 Crotonyl fentanyl (CRM) 25577 Flualprazolam (CRM) 23603 Cyclopropyl fentanyl (hydrochloride) (CRM) 26352 Flubromazolam (CRM) ISO60197 Fentanyl (hydrochloride) (CRM) 18160 Flurazepam (CRM) 25615 β-methyl Fentanyl (hydrochloride) (CRM) 19391 Midazolam (CRM) 19795 FIBF (hydrochloride) (CRM) 19206 Nimetazepam (CRM) 19826 para-Fluorobutyryl fentanyl (hydrochloride) (CRM) ISO60172 Phenazepam (CRM) 19633 Furanyl fentanyl (hydrochloride) (CRM) ISO60182 Temazepam (CRM) ISO60187 Heroin (CRM) -

Appendix 1 Cross-Reference of Research, Generic and Trade Names of Benzodiazepines
Appendix 1 Cross-Reference of Research, Generic and Trade Names of Benzodiazepines Table 1. Benzodiazepine research designations with corresponding generic names Research Designation Generic Name lactam demoxepam methyloxazepam temazepam A 101 nordazepam AB 35616 clorazepate AB 39083 clorazepate AH 3232 clorazepate CB 4261 tetrazepam CB 4306 clorazepate CB 4311 clorazepate CGS 8216 (antagonist) CI683 ripazepam CS 370 cloxazolam CS 430 haloxazolam D40TA estazolam EGYT 341 tofisopam ER 115 temazepam HR 158 loprazolam HR376 clobazam HR 4723 clobazam HR930 fosazepam K3917 temazepam 287 THE BENZODIAZEPINES Research Designation Generic Name LA 111 diazepam LM 2717 clobazam ORF 8063 triflubazam Ro 4-5360 nitrazepam Ro 5-0690 chlordiazepoxide Ro 5-0883 desmethy1chlordiazepoxide Ro 5-2092 demoxepam Ro 5-2180 desmethyldiazepam Ro 5-2807 diazepam Ro 5-2925 desmethylmedazepam Ro 5-3059 nitrazepam Ro 5-3350 bromazepam Ro 5-3438 fludiazepam Ro 5-4023 clonazepam Ro 5-4200 flunitrazepam Ro 5-4556 medazepam Ro 5-5345 temazepam Ro 5-6789 oxazepam Ro 5-6901 flurazepam Ro 15-1788 (antagonist) Ro 21-3981 midazolam RU 31158 loprazolam S 1530 nimetazepam SAH 1123 isoquinazepam SAH 47603 temazepam SB 5833 camazepam SCH 12041 halazepam SCH 16134 quazepam U 28774 ketazolam U 31889 alprazolam U 33030 triazolam W4020 prazepam We 352 triflubazam 288 RESEARCH, GENERIC AND TRADE NAMES Research Designation Generic Name We 941 brotizolam Wy 2917 temazepam Wy 3467 diazepam Wy 3498 oxazepam Wy 3917 temazepam Wy 4036 lorazepam Wy 4082 lormetazepam Wy 4426 oxazepam Y 6047 -

Drug/Substance Trade Name(S)
A B C D E F G H I J K 1 Drug/Substance Trade Name(s) Drug Class Existing Penalty Class Special Notation T1:Doping/Endangerment Level T2: Mismanagement Level Comments Methylenedioxypyrovalerone is a stimulant of the cathinone class which acts as a 3,4-methylenedioxypyprovaleroneMDPV, “bath salts” norepinephrine-dopamine reuptake inhibitor. It was first developed in the 1960s by a team at 1 A Yes A A 2 Boehringer Ingelheim. No 3 Alfentanil Alfenta Narcotic used to control pain and keep patients asleep during surgery. 1 A Yes A No A Aminoxafen, Aminorex is a weight loss stimulant drug. It was withdrawn from the market after it was found Aminorex Aminoxaphen, Apiquel, to cause pulmonary hypertension. 1 A Yes A A 4 McN-742, Menocil No Amphetamine is a potent central nervous system stimulant that is used in the treatment of Amphetamine Speed, Upper 1 A Yes A A 5 attention deficit hyperactivity disorder, narcolepsy, and obesity. No Anileridine is a synthetic analgesic drug and is a member of the piperidine class of analgesic Anileridine Leritine 1 A Yes A A 6 agents developed by Merck & Co. in the 1950s. No Dopamine promoter used to treat loss of muscle movement control caused by Parkinson's Apomorphine Apokyn, Ixense 1 A Yes A A 7 disease. No Recreational drug with euphoriant and stimulant properties. The effects produced by BZP are comparable to those produced by amphetamine. It is often claimed that BZP was originally Benzylpiperazine BZP 1 A Yes A A synthesized as a potential antihelminthic (anti-parasitic) agent for use in farm animals.