996 Anxiolytic Sedatives Hypnotics and Antipsychotics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

986 Anxiolytic Sedatives Hypnotics and Antipsychotics
986 Anxiolytic Sedatives Hypnotics and Antipsychotics Cyclobarbital (BAN, rINN) Detomidine Hydrochloride (BANM, USAN, rINNM) for the sedation of mechanically ventilated patients in intensive care. Dexmedetomidine is given as the hydrochloride, but doses Ciclobarbital; Cyclobarbitalum; Cyclobarbitone; Cyklobarbital; Demotidini Hydrochloridum; Detomidiinihydrokloridi; Detomi- are expressed in terms of the base. Dexmedetomidine hydrochlo- din hydrochlorid; Détomidine, chlorhydrate de; Detomidin-hid- Ethylhexabital; Hexemalum; Syklobarbitaali. 5-(Cyclohex-1-enyl)- ride 118 micrograms is equivalent to about 100 micrograms of 5-ethylbarbituric acid. roklorid; Detomidinhydroklorid; Detomidini hydrochloridum; dexmedetomidine. Hidrocloruro de detomidina; MPV-253-AII. 4-(2,3-Dimethylben- Циклобарбитал It is given in sodium chloride 0.9% by intravenous infusion in a zyl)imidazole hydrochloride. C12H16N2O3 = 236.3. loading dose equivalent to 1 microgram/kg of dexmedetomidine CAS — 52-31-3. Детомидина Гидрохлорид over 10 minutes, followed by a maintenance infusion of 0.2 to ATC — N05C A10. C12H14N2,HCl = 222.7. 0.7 micrograms/kg per hour for up to 24 hours. Reduced doses ATC Vet — QN05C A10. CAS — 76631-46-4 (detomidine); 90038-01-0 (detomi- may be necessary in patients with hepatic or renal impairment, or dine hydrochloride). in the elderly. The racemate, medetomidine (p.1006), is used as the hydrochlo- H ride in veterinary medicine. O N O CH3 ◊ References. N CH3 H3C 1. Venn RM, et al. Preliminary UK experience of dexmedetomi- NH dine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia 1999; 54: 1136–42. HN 2. Bhana N, et al. Dexmedetomidine. Drugs 2000; 59: 263–8. O 3. Coursin DB, et al. Dexmedetomidine. Curr Opin Crit Care (detomidine) 2001; 7: 221–6. -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -
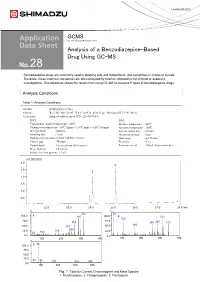
Analysis of a Benzodiazepine-Based Drug Using GC-MS 28
LAAN-E-MS-E028 GCMS Gas Chromatograph Mass Spectrometer Analysis of a Benzodiazepine-Based Drug Using GC-MS 28 Benzodiazepine drugs are commonly used in sleeping aids and tranquilizers, and sometimes in crimes or suicide. Therefore, these chemical substances are often analyzed by forensic laboratories for criminal or academic investigations. This datasheet shows the results from using GC-MS to measure 9 types of benzodiazepine drugs. Analysis Conditions Table 1: Analysis Conditions GC-MS :GCMS-QP2010 Ultra Column : Rxi®-5Sil MS (30 mL. X 0.25 mmI.D., df=0.25 µm, Shimadzu GLC P/N:13623) Glass insert :Silanized splitless insert (P/N: 221-48876-03) [GC] [MS] Vaporization chamber temperature : 260℃ Interface temperature : 280℃ Column oven temperature : 60℃ (2min) -> (10℃/min) -> 320℃ (10min) Ion source temperature : 200℃ Injection mode : Splitless Solvent elution time : 2.0 min Sampling time : 1 min Measurement mode : Scan High pressure injection method: 250 kPa (1.5 min) Mass range : m/z 35-600 Carrier gas : Helium Event time : 0.3 sec Control mode :Linear velocity (45.6 cm/sec) Emission current : 150 µA (high sensitivity) Purge flow rate :3.0 ml/min Sample injection quantity :1.0 µL (x1,000,000) 3.0 3 2.5 2 2.0 1.5 1.0 1 0.5 22.0 23.0 24.0 25.0 26.0 27.0 28.0 min % % 312 55 100.0 1 285 100.0 2 313 109 75.0 75.0 266 259287 342 166 50.0 238 50.0 248 25.0 183 25.0 63 109 0.0 0.0 100 200 300 400 100 200 300 400 % 86 100.0 3 75.0 50.0 25.0 58 99 183 315 387 0.0 100 200 300 400 Fig. -

Behandling Av Ångestsyndrom
Behandling av ångestsyndrom En systematisk litteraturöversikt Volym 2 September 2005 SBU • Statens beredning för medicinsk utvärdering The Swedish Council on Technology Assessment in Health Care SBU utvärderar sjukvårdens metoder SBU (Statens beredning för medicinsk utvärdering) är en statlig myn- Behandling av ångestsyndrom dighet som utvärderar sjukvårdens metoder. SBU analyserar nytta och kostnader för olika medicinska metoder och jämför vetenskapens stånd- punkt med svensk vårdpraxis. Målet är ett bättre beslutsunderlag för En systematisk litteraturöversikt alla som avgör vilken sjukvård som ska bedrivas. Välkommen att besöka SBU:s hemsida, www.sbu.se Volym 2 SBU ger ut tre serier av rapporter. I den första serien presenteras utvär- deringar som utförts av SBU:s projektgrupper. Dessa utvärderingar Projektgrupp åtföljs alltid av en sammanfattning och slutsatser fastställda av SBU:s Lars von Knorring Ingrid Håkanson styrelse och råd. Denna rapportserie ges ut med gula omslag. I den andra (ordförande) (projektassistent) serien, med vita omslag, presenteras aktuella kunskaper inom något Viveka Alton Lundberg Agneta Pettersson område av sjukvården där behov av utvärdering kan föreligga. Den tredje Vanna Beckman (projektledare under serien, Alert-rapporterna, avser tidiga bedömningar av nya metoder inom (deltog 1995–2002) perioden 2004–2005) hälso- och sjukvården. Susanne Bejerot Per-Anders Rydelius Roland Berg Sten Thelander (deltog 1995–2002) (projektledare under Cecilia Björkelund perioden 1995–2004) Per Carlsson Helene Törnqvist (deltog 1995–1999) (deltog 1999–2005) Elias Eriksson Kristian Wahlbeck (deltog 1995–2001) (deltog 2002–2005) Tom Fahlén Hans Ågren Mats Fredrikson Rapporten ”Behandling av ångestsyndrom” består av två volymer (nr 171/1+2) och kan beställas från: Externa granskare SBU, Box 5650, 114 86 Stockholm Fredrik Almqvist Per Høglend Besöksadress: Tyrgatan 7 Alv A. -

Recommended Methods for the Identification and Analysis of Fentanyl and Its Analogues in Biological Specimens
Recommended methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2017 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/53 Original language: English © United Nations, November 2017. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. Barry Logan, Center for Forensic Science Research and Education, at the Fredric Rieders Family Founda- tion and NMS Labs, United States; Amanda L.A. -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

(12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness Et Al
USOO6264,917B1 (12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness et al. (45) Date of Patent: Jul. 24, 2001 (54) TARGETED ULTRASOUND CONTRAST 5,733,572 3/1998 Unger et al.. AGENTS 5,780,010 7/1998 Lanza et al. 5,846,517 12/1998 Unger .................................. 424/9.52 (75) Inventors: Jo Klaveness; Pál Rongved; Dagfinn 5,849,727 12/1998 Porter et al. ......................... 514/156 Lovhaug, all of Oslo (NO) 5,910,300 6/1999 Tournier et al. .................... 424/9.34 FOREIGN PATENT DOCUMENTS (73) Assignee: Nycomed Imaging AS, Oslo (NO) 2 145 SOS 4/1994 (CA). (*) Notice: Subject to any disclaimer, the term of this 19 626 530 1/1998 (DE). patent is extended or adjusted under 35 O 727 225 8/1996 (EP). U.S.C. 154(b) by 0 days. WO91/15244 10/1991 (WO). WO 93/20802 10/1993 (WO). WO 94/07539 4/1994 (WO). (21) Appl. No.: 08/958,993 WO 94/28873 12/1994 (WO). WO 94/28874 12/1994 (WO). (22) Filed: Oct. 28, 1997 WO95/03356 2/1995 (WO). WO95/03357 2/1995 (WO). Related U.S. Application Data WO95/07072 3/1995 (WO). (60) Provisional application No. 60/049.264, filed on Jun. 7, WO95/15118 6/1995 (WO). 1997, provisional application No. 60/049,265, filed on Jun. WO 96/39149 12/1996 (WO). 7, 1997, and provisional application No. 60/049.268, filed WO 96/40277 12/1996 (WO). on Jun. 7, 1997. WO 96/40285 12/1996 (WO). (30) Foreign Application Priority Data WO 96/41647 12/1996 (WO). -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

124.210 Schedule IV — Substances Included. 1
1 CONTROLLED SUBSTANCES, §124.210 124.210 Schedule IV — substances included. 1. Schedule IV shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. 2. Narcotic drugs. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation containing any of the following narcotic drugs, or their salts calculated as the free anhydrous base or alkaloid, in limited quantities as set forth below: a. Not more than one milligram of difenoxin and not less than twenty-five micrograms of atropine sulfate per dosage unit. b. Dextropropoxyphene (alpha-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2- propionoxybutane). c. 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol, its salts, optical and geometric isomers and salts of these isomers (including tramadol). 3. Depressants. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances, including its salts, isomers, and salts of isomers whenever the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation: a. Alprazolam. b. Barbital. c. Bromazepam. d. Camazepam. e. Carisoprodol. f. Chloral betaine. g. Chloral hydrate. h. Chlordiazepoxide. i. Clobazam. j. Clonazepam. k. Clorazepate. l. Clotiazepam. m. Cloxazolam. n. Delorazepam. o. Diazepam. p. Dichloralphenazone. q. Estazolam. r. Ethchlorvynol. s. Ethinamate. t. Ethyl Loflazepate. u. Fludiazepam. v. Flunitrazepam. w. Flurazepam. x. Halazepam. y. Haloxazolam. z. Ketazolam. aa. Loprazolam. ab. Lorazepam. ac. Lormetazepam. ad. Mebutamate. ae. Medazepam. af. Meprobamate. ag. Methohexital. ah. Methylphenobarbital (mephobarbital). -

S1 Table. List of Medications Analyzed in Present Study Drug
S1 Table. List of medications analyzed in present study Drug class Drugs Propofol, ketamine, etomidate, Barbiturate (1) (thiopental) Benzodiazepines (28) (midazolam, lorazepam, clonazepam, diazepam, chlordiazepoxide, oxazepam, potassium Sedatives clorazepate, bromazepam, clobazam, alprazolam, pinazepam, (32 drugs) nordazepam, fludiazepam, ethyl loflazepate, etizolam, clotiazepam, tofisopam, flurazepam, flunitrazepam, estazolam, triazolam, lormetazepam, temazepam, brotizolam, quazepam, loprazolam, zopiclone, zolpidem) Fentanyl, alfentanil, sufentanil, remifentanil, morphine, Opioid analgesics hydromorphone, nicomorphine, oxycodone, tramadol, (10 drugs) pethidine Acetaminophen, Non-steroidal anti-inflammatory drugs (36) (celecoxib, polmacoxib, etoricoxib, nimesulide, aceclofenac, acemetacin, amfenac, cinnoxicam, dexibuprofen, diclofenac, emorfazone, Non-opioid analgesics etodolac, fenoprofen, flufenamic acid, flurbiprofen, ibuprofen, (44 drugs) ketoprofen, ketorolac, lornoxicam, loxoprofen, mefenamiate, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, pranoprofen, proglumetacin, sulindac, talniflumate, tenoxicam, tiaprofenic acid, zaltoprofen, morniflumate, pelubiprofen, indomethacin), Anticonvulsants (7) (gabapentin, pregabalin, lamotrigine, levetiracetam, carbamazepine, valproic acid, lacosamide) Vecuronium, rocuronium bromide, cisatracurium, atracurium, Neuromuscular hexafluronium, pipecuronium bromide, doxacurium chloride, blocking agents fazadinium bromide, mivacurium chloride, (12 drugs) pancuronium, gallamine, succinylcholine -

Acamprosate Pharmaceutical Form(S)/Strength: Gastro-Resistant Tablet P-RMS: IE/H/PSUR/0011/002 Date of FAR: 09.02.2012 4.3 Contraindications
Core Safety Profile Active substance: Acamprosate Pharmaceutical form(s)/strength: Gastro-resistant tablet P-RMS: IE/H/PSUR/0011/002 Date of FAR: 09.02.2012 4.3 Contraindications {Tradename} is contraindicated in patients with hypersensitivity to acamprosate or to any of the excipients. Acamprosate is contraindicated in patients with renal impairment (serum creatinine >120 micromol/l), breastfeeding women (see section 4.6) 4.4 Special warnings and precautions for use The safety and efficacy of {Tradename} has not been established in patients younger than 18 years or older than 65 years. {Tradename} is therefore not recommended for use in these populations. The safety and efficacy of {Tradename} has not been established in patients with severe liver insufficiency (Childs-Pugh Classification C). Because the interrelationship between alcohol dependence, depression and suicidality is well recognised and complex, it is recommended that alcohol-dependent patients, including those treated with acamprosate, be monitored for respective symptoms. Abuse and dependence Non-clinical studies suggest that acamprosate has little or no abuse potential. No evidence of dependence on acamprosate was found in any clinical study thus demonstrating that acamprosate has no significant dependence potential. 4.5 Interaction with other medicinal products and other forms of interaction No change in the frequency of clinical and/or biological adverse reactions has been shown when acamprosate is used concomitantly with disulfiram, oxazepam, tetrabamate or meprobamate. In clinical trials, acamprosate has been safely administered in combination with antidepressants, anxiolytics, hypnotics and sedatives, and non-opioid analgesics. The concomitant intake of alcohol and {Tradename} does not affect the pharmacokinetics of either alcohol or acamprosate. -

Report on the Investigation Results
Pharmaceuticals and Medical Devices Agency This English version is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. Report on the Investigation Results February 28, 2017 Pharmaceuticals and Medical Devices Agency I. Overview of Product [Non-proprietary name] See Attachment 1 [Brand name] See Attachment 1 [Approval holder] See Attachment 1 [Indications] See Attachment 1 [Dosage and administration] See Attachment 1 [Investigating office] Office of Safety II 1 Pharmaceuticals and Medical Devices Agency This English version is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. II. Background of the investigation 1. Status in Japan Hypnotics and anxiolytics are prescribed by various specialties and widely used in clinical practice. In particular, benzodiazepine (BZ) receptor agonists, which act on BZ receptors, bind to gamma-aminobutyric acid (GABA)A-BZ receptor complex and enhance the function of GABAA receptors. This promotes neurotransmission of inhibitory systems and demonstrates hypnotic/sedative effects, anxiolytic effects, muscle relaxant effects, and antispasmodic effects. Since the approval of chlordiazepoxide in March 1961, many BZ receptor agonists have been approved as hypnotics and anxiolytics. Currently, hypnotics and anxiolytics are causative agents of drug-related disorders such as drug dependence in Japanese clinical practice. Hypnotics and anxiolitics that rank high in causative agents are BZ receptor agonists for which high frequencies of high doses and multidrug prescriptions have been reported (Japanese Journal of Clinical Psychopharmacology 2013; 16(6): 803-812, Modern Physician 2014; 34(6): 653-656, etc.).