II.3.4 Benzodiazepines by Hiroshi Seno and Hideki Hattori
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GABA Receptors
D Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews Review No.7 / 1-2011 GABA receptors Wolfgang Froestl , CNS & Chemistry Expert, AC Immune SA, PSE Building B - EPFL, CH-1015 Lausanne, Phone: +41 21 693 91 43, FAX: +41 21 693 91 20, E-mail: [email protected] GABA Activation of the GABA A receptor leads to an influx of chloride GABA ( -aminobutyric acid; Figure 1) is the most important and ions and to a hyperpolarization of the membrane. 16 subunits with γ most abundant inhibitory neurotransmitter in the mammalian molecular weights between 50 and 65 kD have been identified brain 1,2 , where it was first discovered in 1950 3-5 . It is a small achiral so far, 6 subunits, 3 subunits, 3 subunits, and the , , α β γ δ ε θ molecule with molecular weight of 103 g/mol and high water solu - and subunits 8,9 . π bility. At 25°C one gram of water can dissolve 1.3 grams of GABA. 2 Such a hydrophilic molecule (log P = -2.13, PSA = 63.3 Å ) cannot In the meantime all GABA A receptor binding sites have been eluci - cross the blood brain barrier. It is produced in the brain by decarb- dated in great detail. The GABA site is located at the interface oxylation of L-glutamic acid by the enzyme glutamic acid decarb- between and subunits. Benzodiazepines interact with subunit α β oxylase (GAD, EC 4.1.1.15). It is a neutral amino acid with pK = combinations ( ) ( ) , which is the most abundant combi - 1 α1 2 β2 2 γ2 4.23 and pK = 10.43. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -
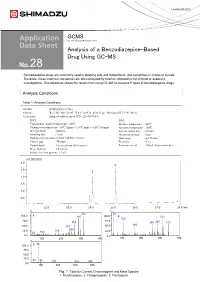
Analysis of a Benzodiazepine-Based Drug Using GC-MS 28
LAAN-E-MS-E028 GCMS Gas Chromatograph Mass Spectrometer Analysis of a Benzodiazepine-Based Drug Using GC-MS 28 Benzodiazepine drugs are commonly used in sleeping aids and tranquilizers, and sometimes in crimes or suicide. Therefore, these chemical substances are often analyzed by forensic laboratories for criminal or academic investigations. This datasheet shows the results from using GC-MS to measure 9 types of benzodiazepine drugs. Analysis Conditions Table 1: Analysis Conditions GC-MS :GCMS-QP2010 Ultra Column : Rxi®-5Sil MS (30 mL. X 0.25 mmI.D., df=0.25 µm, Shimadzu GLC P/N:13623) Glass insert :Silanized splitless insert (P/N: 221-48876-03) [GC] [MS] Vaporization chamber temperature : 260℃ Interface temperature : 280℃ Column oven temperature : 60℃ (2min) -> (10℃/min) -> 320℃ (10min) Ion source temperature : 200℃ Injection mode : Splitless Solvent elution time : 2.0 min Sampling time : 1 min Measurement mode : Scan High pressure injection method: 250 kPa (1.5 min) Mass range : m/z 35-600 Carrier gas : Helium Event time : 0.3 sec Control mode :Linear velocity (45.6 cm/sec) Emission current : 150 µA (high sensitivity) Purge flow rate :3.0 ml/min Sample injection quantity :1.0 µL (x1,000,000) 3.0 3 2.5 2 2.0 1.5 1.0 1 0.5 22.0 23.0 24.0 25.0 26.0 27.0 28.0 min % % 312 55 100.0 1 285 100.0 2 313 109 75.0 75.0 266 259287 342 166 50.0 238 50.0 248 25.0 183 25.0 63 109 0.0 0.0 100 200 300 400 100 200 300 400 % 86 100.0 3 75.0 50.0 25.0 58 99 183 315 387 0.0 100 200 300 400 Fig. -

Benzodiazepine Group ELISA Kit
Benzodiazepine Group ELISA Kit Benzodiazepine Background Since their introduction in the 1960s, benzodiazepines have been widely prescribed for the treatment of anxiety, insomnia, muscle spasms, alcohol withdrawal, and seizure-prevention as they are depressants of the central nervous system. Despite the fact that they are highly effective for their intended use, benzodiazepines are prescribed with caution as they can be highly addictive. In fact, researchers at NIDA (National Institute on Drug Abuse) have shown that addiction for benzodiazepines is similar to that of opioids, cannabinoids, and GHB. Common street names of benzodiazepines include “Benzos” and “Downers”. The five most encountered benzodiazepines on the illicit market are alprazolam (Xanax), lorazepam (Ativan), clonazepam (Klonopin), diazepam (Valium), and temazepam (Restori). The method of abuse is typically oral or snorted in crushed form. The DEA notes a particularly high rate of abuse among heroin and cocaine abusers. Designer benzodiazepines are currently offered in online shops selling “research chemicals”, providing drug abusers an alternative to prescription-only benzodiazepines. Data defining pharmacokinetic parameters, drug metabolisms, and detectability in biological fluids is limited. This lack of information presents a challenge to forensic laboratories. Changes in national narcotics laws in many countries led to the control of (phenazepam and etizolam), which were marketed by pharmaceutical companies in some countries. With the control of phenazepam and etizolam, clandestine laboratories have begun researching and manufacturing alternative benzodiazepines as legal substitutes. Delorazepam, diclazepam, pyrazolam, and flubromazepam have emerged as compounds in this class of drugs. References Drug Enforcement Administration, Office of Diversion Control. “Benzodiazepines.” http://www.deadiversion.usdoj.gov/drugs_concern/benzo_1. -

Recommended Methods for the Identification and Analysis of Fentanyl and Its Analogues in Biological Specimens
Recommended methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2017 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/53 Original language: English © United Nations, November 2017. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. Barry Logan, Center for Forensic Science Research and Education, at the Fredric Rieders Family Founda- tion and NMS Labs, United States; Amanda L.A. -

Control Substance List
Drugs DrugID SubstanceName DEANumbScheNarco OtherNames 1 1-(1-Phenylcyclohexyl)pyrrolidine 7458 I N PCPy, PHP, rolicyclidine 2 1-(2-Phenylethyl)-4-phenyl-4-acetoxypiperidine 9663 I Y PEPAP, synthetic heroin 3 1-[1-(2-Thienyl)cyclohexyl]piperidine 7470 I N TCP, tenocyclidine 4 1-[1-(2-Thienyl)cyclohexyl]pyrrolidine 7473 I N TCPy 5 13Beta-ethyl-17beta-hydroxygon-4-en-3-one 4000 III N 6 17Alpha-methyl-3alpha,17beta-dihydroxy-5alpha-androstane 4000 III N 7 17Alpha-methyl-3beta,17beta-dihydroxy-5alpha-androstane 4000 III N 8 17Alpha-methyl-3beta,17beta-dihydroxyandrost-4-ene 4000 III N 9 17Alpha-methyl-4-hydroxynandrolone (17alpha-methyl-4-hyd 4000 III N 10 17Alpha-methyl-delta1-dihydrotestosterone (17beta-hydroxy- 4000 III N 17-Alpha-methyl-1-testosterone 11 19-Nor-4-androstenediol (3beta,17beta-dihydroxyestr-4-ene; 4000 III N 12 19-Nor-4-androstenedione (estr-4-en-3,17-dione) 4000 III N 13 19-Nor-5-androstenediol (3beta,17beta-dihydroxyestr-5-ene; 4000 III N 14 19-Nor-5-androstenedione (estr-5-en-3,17-dione) 4000 III N 15 1-Androstenediol (3beta,17beta-dihydroxy-5alpha-androst-1- 4000 III N 16 1-Androstenedione (5alpha-androst-1-en-3,17-dione) 4000 III N 17 1-Methyl-4-phenyl-4-propionoxypiperidine 9661 I Y MPPP, synthetic heroin 18 1-Phenylcyclohexylamine 7460 II N PCP precursor 19 1-Piperidinocyclohexanecarbonitrile 8603 II N PCC, PCP precursor 20 2,5-Dimethoxy-4-(n)-propylthiophenethylamine 7348 I N 2C-T-7 21 2,5-Dimethoxy-4-ethylamphetamine 7399 I N DOET 22 2,5-Dimethoxyamphetamine 7396 I N DMA, 2,5-DMA 23 3,4,5-Trimethoxyamphetamine -

124.210 Schedule IV — Substances Included. 1
1 CONTROLLED SUBSTANCES, §124.210 124.210 Schedule IV — substances included. 1. Schedule IV shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. 2. Narcotic drugs. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation containing any of the following narcotic drugs, or their salts calculated as the free anhydrous base or alkaloid, in limited quantities as set forth below: a. Not more than one milligram of difenoxin and not less than twenty-five micrograms of atropine sulfate per dosage unit. b. Dextropropoxyphene (alpha-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2- propionoxybutane). c. 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol, its salts, optical and geometric isomers and salts of these isomers (including tramadol). 3. Depressants. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances, including its salts, isomers, and salts of isomers whenever the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation: a. Alprazolam. b. Barbital. c. Bromazepam. d. Camazepam. e. Carisoprodol. f. Chloral betaine. g. Chloral hydrate. h. Chlordiazepoxide. i. Clobazam. j. Clonazepam. k. Clorazepate. l. Clotiazepam. m. Cloxazolam. n. Delorazepam. o. Diazepam. p. Dichloralphenazone. q. Estazolam. r. Ethchlorvynol. s. Ethinamate. t. Ethyl Loflazepate. u. Fludiazepam. v. Flunitrazepam. w. Flurazepam. x. Halazepam. y. Haloxazolam. z. Ketazolam. aa. Loprazolam. ab. Lorazepam. ac. Lormetazepam. ad. Mebutamate. ae. Medazepam. af. Meprobamate. ag. Methohexital. ah. Methylphenobarbital (mephobarbital). -

S1 Table. List of Medications Analyzed in Present Study Drug
S1 Table. List of medications analyzed in present study Drug class Drugs Propofol, ketamine, etomidate, Barbiturate (1) (thiopental) Benzodiazepines (28) (midazolam, lorazepam, clonazepam, diazepam, chlordiazepoxide, oxazepam, potassium Sedatives clorazepate, bromazepam, clobazam, alprazolam, pinazepam, (32 drugs) nordazepam, fludiazepam, ethyl loflazepate, etizolam, clotiazepam, tofisopam, flurazepam, flunitrazepam, estazolam, triazolam, lormetazepam, temazepam, brotizolam, quazepam, loprazolam, zopiclone, zolpidem) Fentanyl, alfentanil, sufentanil, remifentanil, morphine, Opioid analgesics hydromorphone, nicomorphine, oxycodone, tramadol, (10 drugs) pethidine Acetaminophen, Non-steroidal anti-inflammatory drugs (36) (celecoxib, polmacoxib, etoricoxib, nimesulide, aceclofenac, acemetacin, amfenac, cinnoxicam, dexibuprofen, diclofenac, emorfazone, Non-opioid analgesics etodolac, fenoprofen, flufenamic acid, flurbiprofen, ibuprofen, (44 drugs) ketoprofen, ketorolac, lornoxicam, loxoprofen, mefenamiate, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, pranoprofen, proglumetacin, sulindac, talniflumate, tenoxicam, tiaprofenic acid, zaltoprofen, morniflumate, pelubiprofen, indomethacin), Anticonvulsants (7) (gabapentin, pregabalin, lamotrigine, levetiracetam, carbamazepine, valproic acid, lacosamide) Vecuronium, rocuronium bromide, cisatracurium, atracurium, Neuromuscular hexafluronium, pipecuronium bromide, doxacurium chloride, blocking agents fazadinium bromide, mivacurium chloride, (12 drugs) pancuronium, gallamine, succinylcholine -

Drug Screening: Actual Status, Pitfalls and Suggestions for Improvement Drogenscreening: Gegenwa¨ Rtiger Stand, Fehlermo¨ Glichkeiten Und Verbesserungsvorschla¨Ge
J Lab Med 2004;28(4):317–325 ᮊ 2004 by Walter de Gruyter • Berlin • New York 2004/03304 Drug Monitoring und Toxikologie Redaktion: V. W. Armstrong Drug screening: Actual status, pitfalls and suggestions for improvement Drogenscreening: Gegenwa¨ rtiger Stand, Fehlermo¨ glichkeiten und Verbesserungsvorschla¨ge Wolf R. Ku¨ lpmann* pretierbar. In Wirklichkeit mu¨ ssen viele Aspekte beru¨ ck- sichtigt werden, um einen aussagekra¨ ftigen Befund zu Klinische Chemie, Medizinische Hochschule Hannover, erhalten, z.B. Pra¨ analytik, Spezifita¨ t und Empfindlichkeit Hannover, Germany des Verfahrens oder Qualita¨ tssicherung. Obwohl die Abstract mechanisierten Meßverfahren naturgema¨ ß quantitative Ergebnisse liefern, wird aufgezeigt, daß es sich in der Immunoassays for drug screening are often regarded as Regel empfiehlt, qualitative Befunde zu erstellen. Die procedures which are easily performed and interpreted. Verfahren erlauben aber im Gegensatz zu den auch fu¨r But in fact, many aspects have to be considered to diese Zwecke verwendeten Teststreifen die Entschei- obtain a meaningful result. Among these are sampling, dungsgrenze (cut-off) der jeweiligen Fragestellung anzu- specificity and sensitivity of assays, and quality assess- passen, z.B. bei akuter Intoxikation, chronischem Abusus ment. Although the mechanised procedures yield quan- oder U¨ berwachung des Drogenentzugs. titative results, there are good reasons to generally report Ein schwerwiegender Nachteil von Immunoassays zum qualitative findings. The mechanised procedures allow Nachweis von Amphetamin und a¨ hnlichen Substanzen, the adjustment of the cut-off concentration to the clinical Barbituraten, Benzodiazepinen und Opiaten besteht dar- setting, e.g. in the case of acute intoxication, chronic in, daß eine starke Kreuzreaktivita¨ t des Antiko¨ rpers abuse or drug withdrawal, an important advantage as gegenu¨ ber pharmakologisch wenig wirksamen Verbin- compared to test strips. -

Statistical Analysis Plan Statistical Center for HIV/AIDS Research & SCHARP Prevention SD Standard Deviation SI International System of Units
67$7,67,&$/$1$/<6,63/$1 $:((.)/(;,%/('26(23(1/$%(/ 08/7,&(17(5(;7(16,21678'<72(9$/8$7(7+( /21*7(506$)(7<$1'())(&7,9(1(662) /85$6,'21(,13(',$75,&68%-(&76 /XUDVLGRQH60/DWXGD 6WXG\1R' 9HUVLRQ1XPEHU)LQDO 9HUVLRQ'DWH1RY $XWKRUL]DWLRQ6LJQDWXUH3DJH $:((.)/(;,%/('26(23(1/$%(/08/7,&(17(5(;7(16,21 678'<72(9$/8$7(7+(/21*7(506$)(7<$1'())(&7,9(1(662) /85$6,'21(,13(',$75,&68%-(&76 $XWKRU 1DPH 'DWH 3RVLWLRQ6HQLRU 'LUHFWRU%LRVWDWLVWLFV &RPSDQ\6XQRYLRQ3KDUPDFHXWLFDOV,QF $SSURYHGE\ 1DPH 'DWH 3RVLWLRQ 6HQLRU'LUHFWRU&OLQLFDO'HYHORSPHQW DQG0HGLFDO$IIDLUV&16 &RPSDQ\6XQRYLRQ3KDUPDFHXWLFDOV,QF 1DPH 'DWH 3RVLWLRQ([HFXWLYH'LUHFWRU%LRVWDWLVWLFV &RPSDQ\6XQRYLRQ3KDUPDFHXWLFDOV,QF Table of contents 1. INTRODUCTION ........................................................................................................9 1.1. Study Objectives ...........................................................................................................9 1.2. Study Design ...............................................................................................................10 1.2.1. Determination of Sample Size ....................................................................................11 1.2.2. Randomization and Blinding ......................................................................................11 2. ANALYSES PLANNED ............................................................................................12 2.1. General Analysis Definition .......................................................................................12 2.1.1. Logic -

(12) Patent Application Publication (10) Pub. No.: US 2009/0005722 A1 Jennings-Spring (43) Pub
US 20090005722A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0005722 A1 Jennings-Spring (43) Pub. Date: Jan. 1, 2009 (54) SKIN-CONTACTING-ADHESIVE FREE Publication Classification DRESSING (51) Int. Cl. Inventor: Barbara Jennings-Spring, Jupiter, A61N L/30 (2006.01) (76) A6F I3/00 (2006.01) FL (US) A6IL I5/00 (2006.01) Correspondence Address: AOIG 7/06 (2006.01) Irving M. Fishman AOIG 7/04 (2006.01) c/o Cohen, Tauber, Spievack and Wagner (52) U.S. Cl. .................. 604/20: 602/43: 602/48; 4771.5; Suite 2400, 420 Lexington Avenue 47/13 New York, NY 10170 (US) (57) ABSTRACT (21) Appl. No.: 12/231,104 A dressing having a flexible sleeve shaped to accommodate a Substantially cylindrical body portion, the sleeve having a (22) Filed: Aug. 29, 2008 lining which is substantially non-adherent to the body part being bandaged and having a peripheral securement means Related U.S. Application Data which attaches two peripheral portions to each other without (63) Continuation-in-part of application No. 1 1/434,689, those portions being circumferentially adhered to the sleeve filed on May 16, 2006. portion. Patent Application Publication Jan. 1, 2009 Sheet 1 of 9 US 2009/0005722 A1 Patent Application Publication Jan. 1, 2009 Sheet 2 of 9 US 2009/0005722 A1 10 8 F.G. 5 Patent Application Publication Jan. 1, 2009 Sheet 3 of 9 US 2009/0005722 A1 13 FIG.6 2 - Y TIII Till "T fift 11 10 FIG.7 8 13 6 - 12 - Timir" "in "in "MINIII. -

Introduced B.,Byhansen, 16
LB301 LB301 2021 2021 LEGISLATURE OF NEBRASKA ONE HUNDRED SEVENTH LEGISLATURE FIRST SESSION LEGISLATIVE BILL 301 Introduced by Hansen, B., 16. Read first time January 12, 2021 Committee: Judiciary 1 A BILL FOR AN ACT relating to the Uniform Controlled Substances Act; to 2 amend sections 28-401, 28-405, and 28-416, Revised Statutes 3 Cumulative Supplement, 2020; to redefine terms; to change drug 4 schedules and adopt federal drug provisions; to change a penalty 5 provision; and to repeal the original sections. 6 Be it enacted by the people of the State of Nebraska, -1- LB301 LB301 2021 2021 1 Section 1. Section 28-401, Revised Statutes Cumulative Supplement, 2 2020, is amended to read: 3 28-401 As used in the Uniform Controlled Substances Act, unless the 4 context otherwise requires: 5 (1) Administer means to directly apply a controlled substance by 6 injection, inhalation, ingestion, or any other means to the body of a 7 patient or research subject; 8 (2) Agent means an authorized person who acts on behalf of or at the 9 direction of another person but does not include a common or contract 10 carrier, public warehouse keeper, or employee of a carrier or warehouse 11 keeper; 12 (3) Administration means the Drug Enforcement Administration of the 13 United States Department of Justice; 14 (4) Controlled substance means a drug, biological, substance, or 15 immediate precursor in Schedules I through V of section 28-405. 16 Controlled substance does not include distilled spirits, wine, malt 17 beverages, tobacco, hemp, or any nonnarcotic substance if such substance 18 may, under the Federal Food, Drug, and Cosmetic Act, 21 U.S.C.