PMDA Alert for Proper Use of Drugs When Using Benzodiazepine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn Et Al
US 2004O224012A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0224012 A1 Suvanprakorn et al. (43) Pub. Date: Nov. 11, 2004 (54) TOPICAL APPLICATION AND METHODS Related U.S. Application Data FOR ADMINISTRATION OF ACTIVE AGENTS USING LIPOSOME MACRO-BEADS (63) Continuation-in-part of application No. 10/264,205, filed on Oct. 3, 2002. (76) Inventors: Pichit Suvanprakorn, Bangkok (TH); (60) Provisional application No. 60/327,643, filed on Oct. Tanusin Ploysangam, Bangkok (TH); 5, 2001. Lerson Tanasugarn, Bangkok (TH); Suwalee Chandrkrachang, Bangkok Publication Classification (TH); Nardo Zaias, Miami Beach, FL (US) (51) Int. CI.7. A61K 9/127; A61K 9/14 (52) U.S. Cl. ............................................ 424/450; 424/489 Correspondence Address: (57) ABSTRACT Eric G. Masamori 6520 Ridgewood Drive A topical application and methods for administration of Castro Valley, CA 94.552 (US) active agents encapsulated within non-permeable macro beads to enable a wider range of delivery vehicles, to provide longer product shelf-life, to allow multiple active (21) Appl. No.: 10/864,149 agents within the composition, to allow the controlled use of the active agents, to provide protected and designable release features and to provide visual inspection for damage (22) Filed: Jun. 9, 2004 and inconsistency. US 2004/0224012 A1 Nov. 11, 2004 TOPCAL APPLICATION AND METHODS FOR 0006 Various limitations on the shelf-life and use of ADMINISTRATION OF ACTIVE AGENTS USING liposome compounds exist due to the relatively fragile LPOSOME MACRO-BEADS nature of liposomes. Major problems encountered during liposome drug Storage in vesicular Suspension are the chemi CROSS REFERENCE TO OTHER cal alterations of the lipoSome compounds, Such as phos APPLICATIONS pholipids, cholesterols, ceramides, leading to potentially toxic degradation of the products, leakage of the drug from 0001) This application claims the benefit of U.S. -

Understanding Benzodiazephine Use, Abuse, and Detection
Siemens Healthcare Diagnostics, the leading clinical diagnostics company, is committed to providing clinicians with the vital information they need for the accurate diagnosis, treatment and monitoring of patients. Our comprehensive portfolio of performance-driven systems, unmatched menu offering and IT solutions, in conjunction with highly responsive service, is designed to streamline workflow, enhance operational efficiency and support improved patient care. Syva, EMIT, EMIT II, EMIT d.a.u., and all associated marks are trademarks of General Siemens Healthcare Diagnostics Inc. All Drugs other trademarks and brands are the Global Division property of their respective owners. of Abuse Siemens Healthcare Product availability may vary from Diagnostics Inc. country to country and is subject 1717 Deerfield Road to varying regulatory requirements. Deerfield, IL 60015-0778 Please contact your local USA representative for availability. www.siemens.com/diagnostics Siemens Global Headquarters Global Siemens Healthcare Headquarters Siemens AG Understanding Wittelsbacherplatz 2 Siemens AG 80333 Muenchen Healthcare Sector Germany Henkestrasse 127 Benzodiazephine Use, 91052 Erlangen Germany Abuse, and Detection Telephone: +49 9131 84 - 0 www.siemens.com/healthcare www.usa.siemens.com/diagnostics Answers for life. Order No. A91DX-0701526-UC1-4A00 | Printed in USA | © 2009 Siemens Healthcare Diagnostics Inc. Syva has been R1 R2 a leading developer N and manufacturer of AB R3 X N drugs-of-abuse tests R4 for more than 30 years. R2 C Now part of Siemens Healthcare ® Diagnostics, Syva boasts a long and Benzodiazepines have as their basic chemical structure successful track record in drugs-of-abuse a benzene ring fused to a seven-membered diazepine ring. testing, and leads the industry in the All important benzodiazepines contain a 5-aryl substituent ring (ring C) and a 1,4–diazepine ring. -

Socio-Demographic and Clinical Characteristics of Benzodiazepine Long-Term Users: Results from a Tertiary Care Center ⁎ F
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Florence Research Available online at www.sciencedirect.com ScienceDirect Comprehensive Psychiatry 69 (2016) 211–215 www.elsevier.com/locate/comppsych Socio-demographic and clinical characteristics of benzodiazepine long-term users: Results from a tertiary care center ⁎ F. Coscia, , G. Mansuetoa, M. Faccinib, R. Casarib, F. Lugobonib aDepartment of Health Sciences, University of Florence, via di San Salvi 12, 50135, Florence, Italy bAddiction Unit, Verona University Hospital, piazzale Aristide Stefani 1, 37126, Verona, Italy Abstract Objective: The use of benzodiazepines (BDZs) represents a critical issue since a long-term treatment may lead to dependence. This study aimed at evaluating socio-demographic and clinical characteristics of BZD long-term users who followed a detoxification program at a tertiary care center. Method: Two hundred-five inpatients were evaluated. Socio-demographic (e.g., gender, age, education) and clinical information (e.g., BZD used, dose, reason of prescription) was collected. BZDs dose was standardized as diazepam dose equivalents and was compared via the Defined Daily Dose (DDD). Chi-square, Fisher test, ANOVA and Bonferroni analyses were performed. Results: Females were more frequently BDZ long-term users than males. Hypnotic BZDs were frequently prescribed for problems different from sleep disturbances. Lorazepam, alprazolam, and lormetazepam were the most prescribed drugs. Lorazepam was more frequently used by males, consumed for a long period, in pills, and prescribed for anxiety. Lormetazepam was more frequently consumed by females with a high school education, having a psychiatric disorder, taken in drops and prescribed for insomnia. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Table 6.12: Deaths from Poisoning, by Sex and Cause, Scotland, 2016
Table 6.12: Deaths from poisoning, by sex and cause, Scotland, 2016 ICD code(s), cause of death and substance(s) 1 Both Males Females ALL DEATHS FROM POISONING 2 1130 766 364 ACCIDENTS 850 607 243 X40 - X49 Accidental poisoning by and exposure to … X40 - Nonopioid analgesics, antipyretics and antirheumatics Paracetamol 2 1 1 Paracetamol, Cocaine, Amphetamine || 1 0 1 X41 - Antiepileptic, sedative-hypnotic, antiparkinsonism and psychotropic drugs, not elsewhere classified Alprazolam, MDMA, Cocaine || Cannabis, Alcohol 1 1 0 Alprazolam, Methadone || Pregabalin, Tramadol, Gabapentin, Cannabis 1 1 0 Alprazolam, Morphine, Heroin, Dihydrocodeine, Buprenorphine || Alcohol 1 1 0 Alprazolam, Oxycodone, Alcohol || Paracetamol 1 0 1 Amitriptyline, Cocaine, Etizolam || Paracetamol, Codeine, Hydrocodone, Alcohol 1 1 0 Amitriptyline, Dihydrocodeine || Diazepam, Paracetamol, Verapamil, Alcohol 1 1 0 Amitriptyline, Fluoxetine, Alcohol 1 0 1 Amitriptyline, Methadone, Diazepam || 1 1 0 Amitriptyline, Methadone, Morphine, Etizolam || Gabapentin, Cannabis, Alcohol 1 1 0 Amitriptyline, Venlafaxine 1 0 1 Amphetamine 1 1 0 Amphetamine || 1 1 0 Amphetamine || Alcohol 1 1 0 Amphetamine || Chlorpromazine 1 1 0 Amphetamine || Fluoxetine 1 1 0 Amphetamine, Dihydrocodeine, Alcohol || Procyclidine, Tramadol, Duloxetine, Haloperidol 1 0 1 Amphetamine, MDMA || Diclazepam, Cannabis, Alcohol 1 1 0 Amphetamine, Methadone || 1 1 0 Amphetamine, Oxycodone, Gabapentin, Zopiclone, Diazepam || Paracetamol, Alcohol 1 0 1 Amphetamine, Tramadol || Mirtazapine, Alcohol 1 1 0 Benzodiazepine -
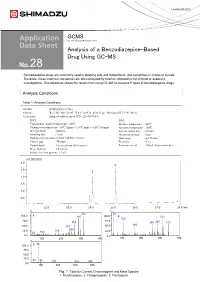
Analysis of a Benzodiazepine-Based Drug Using GC-MS 28
LAAN-E-MS-E028 GCMS Gas Chromatograph Mass Spectrometer Analysis of a Benzodiazepine-Based Drug Using GC-MS 28 Benzodiazepine drugs are commonly used in sleeping aids and tranquilizers, and sometimes in crimes or suicide. Therefore, these chemical substances are often analyzed by forensic laboratories for criminal or academic investigations. This datasheet shows the results from using GC-MS to measure 9 types of benzodiazepine drugs. Analysis Conditions Table 1: Analysis Conditions GC-MS :GCMS-QP2010 Ultra Column : Rxi®-5Sil MS (30 mL. X 0.25 mmI.D., df=0.25 µm, Shimadzu GLC P/N:13623) Glass insert :Silanized splitless insert (P/N: 221-48876-03) [GC] [MS] Vaporization chamber temperature : 260℃ Interface temperature : 280℃ Column oven temperature : 60℃ (2min) -> (10℃/min) -> 320℃ (10min) Ion source temperature : 200℃ Injection mode : Splitless Solvent elution time : 2.0 min Sampling time : 1 min Measurement mode : Scan High pressure injection method: 250 kPa (1.5 min) Mass range : m/z 35-600 Carrier gas : Helium Event time : 0.3 sec Control mode :Linear velocity (45.6 cm/sec) Emission current : 150 µA (high sensitivity) Purge flow rate :3.0 ml/min Sample injection quantity :1.0 µL (x1,000,000) 3.0 3 2.5 2 2.0 1.5 1.0 1 0.5 22.0 23.0 24.0 25.0 26.0 27.0 28.0 min % % 312 55 100.0 1 285 100.0 2 313 109 75.0 75.0 266 259287 342 166 50.0 238 50.0 248 25.0 183 25.0 63 109 0.0 0.0 100 200 300 400 100 200 300 400 % 86 100.0 3 75.0 50.0 25.0 58 99 183 315 387 0.0 100 200 300 400 Fig. -

XANAX® Alprazolam Tablets, USP
XANAX® alprazolam tablets, USP CIV WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death [see Warnings, Drug Interactions]. Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation. DESCRIPTION XANAX Tablets contain alprazolam which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds. The chemical name of alprazolam is 8-Chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-α] [1,4] benzodiazepine. The structural formula is represented to the right: Alprazolam is a white crystalline powder, which is soluble in methanol or ethanol but which has no appreciable solubility in water at physiological pH. Each XANAX Tablet, for oral administration, contains 0.25, 0.5, 1 or 2 mg of alprazolam. XANAX Tablets, 2 mg, are multi-scored and may be divided as shown below: 1 Reference ID: 4029640 Inactive ingredients: Cellulose, corn starch, docusate sodium, lactose, magnesium stearate, silicon dioxide and sodium benzoate. In addition, the 0.5 mg tablet contains FD&C Yellow No. 6 and the 1 mg tablet contains FD&C Blue No. 2. CLINICAL PHARMACOLOGY Pharmacodynamics CNS agents of the 1,4 benzodiazepine class presumably exert their effects by binding at stereo specific receptors at several sites within the central nervous system. Their exact mechanism of action is unknown. Clinically, all benzodiazepines cause a dose-related central nervous system depressant activity varying from mild impairment of task performance to hypnosis. -

Recommended Methods for the Identification and Analysis of Fentanyl and Its Analogues in Biological Specimens
Recommended methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2017 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/53 Original language: English © United Nations, November 2017. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. Barry Logan, Center for Forensic Science Research and Education, at the Fredric Rieders Family Founda- tion and NMS Labs, United States; Amanda L.A. -

Drug Names That Are Too Close for Comfort
MEDICATION SAFETY Drug Names That Are Too Close for Comfort and therefore at risk of “seeing” prescriptions for the newer product clobazam as the more familiar clonazePAM. Use TWO IDENtifieRS at THE POS Within the span of a couple of days, the Institute for Safe Medication Practices received two more reports of wrong patient errors at the point-of-sale. Both were submitted by the parents of the patients involved. In one event, a teenager, who Look- and sound-alike similarity between the official names was supposed to receive methylphenidate for Attention Deficit clobazam (ONFI) and clonazePAM (KLONOPIN) has led Hyperactivity Disorder (ADHD), was given a cardiac drug to several reports expressing concern that patients may in- intended for a different patient. The mother who was picking advertently receive the wrong medication in either hospital up the prescription for her son caught the error because or outpatient settings. The drugs share similar indications, the pharmacist mentioned that “this will help with the chest although they have 10-fold strength difference in available pains.” Of course, this means that the other patient was given dosage forms. Clobazam was approved by Food and Drug the methylphenidate instead of the cardiac drug. It turned out Administration in October 2011 for adjunctive treatment of that the teenager and the other patient had the same name. Lennox-Gaustaut seizures and other epileptic syndromes. Lennox-Gaustaut is a form of childhood-onset epilepsy In the second case, a patient was dispensed amLODIPine, a characterized by frequent seizures and different seizure calcium channel blocker, instead of the anticonvulsant gab- types, often accompanied by developmental delay and apentin. -

Pharmacological Properties of GABAA- Receptors Containing Gamma1
Molecular Pharmacology Fast Forward. Published on November 4, 2005 as DOI: 10.1124/mol.105.017236 Molecular PharmacologyThis article hasFast not Forward.been copyedited Published and formatted. on The November final version 7, may 2005 differ as from doi:10.1124/mol.105.017236 this version. MOLPHARM/2005/017236 Pharmacological properties of GABAA- receptors containing gamma1- subunits Khom S.1, Baburin I.1, Timin EN, Hohaus A., Sieghart W., Hering S. Downloaded from Department of Pharmacology and Toxicology, University of Vienna Center of Brain Research , Medical University of Vienna, Division of Biochemistry and molpharm.aspetjournals.org Molecular Biology at ASPET Journals on September 27, 2021 1 Copyright 2005 by the American Society for Pharmacology and Experimental Therapeutics. Molecular Pharmacology Fast Forward. Published on November 4, 2005 as DOI: 10.1124/mol.105.017236 This article has not been copyedited and formatted. The final version may differ from this version. MOLPHARM/2005/017236 Running Title: GABAA- receptors containing gamma1- subunits Corresponding author: Steffen Hering Department of Pharmacology and Toxicology University of Vienna Althanstrasse 14 Downloaded from A-1090 Vienna Telephone number: +43-1-4277-55301 Fax number: +43-1-4277-9553 molpharm.aspetjournals.org [email protected] Text pages: 29 at ASPET Journals on September 27, 2021 Tables: 2 Figures: 7 References: 26 Abstract: 236 words Introduction:575 words Discussion:1383 words 2 Molecular Pharmacology Fast Forward. Published on November 4, 2005 as DOI: 10.1124/mol.105.017236 This article has not been copyedited and formatted. The final version may differ from this version. MOLPHARM/2005/017236 Abstract GABAA receptors composed of α1, β2, γ1-subunits are expressed in only a few areas of the brain and thus represent interesting drug targets. -

ETIZOLAM Critical Review Report Agenda Item 4.13
ETIZOLAM Critical Review Report Agenda Item 4.13 Expert Committee on Drug Dependence Thirty-ninth Meeting Geneva, 6-10 November 2017 39th ECDD (2017) Agenda item 4.13 Etizolam Page 2 of 20 39th ECDD (2017) Agenda item 4.13 Etizolam Contents Acknowledgements.................................................................................................................................. 5 Summary...................................................................................................................................................... 6 1. Substance identification ....................................................................................................................... 7 A. International Nonproprietary Name (INN).......................................................................................................... 7 B. Chemical Abstract Service (CAS) Registry Number .......................................................................................... 7 C. Other Chemical Names ................................................................................................................................................... 7 D. Trade Names ....................................................................................................................................................................... 7 E. Street Names ....................................................................................................................................................................... 8 F. Physical Appearance -

ETIZOLAM in POST-MORTEM CASES Joanna Hockenhull1
ETIZOLAM IN POST-MORTEM CASES Joanna Hockenhull1 1Toxicology Unit, Faculty of Medicine, Imperial College London. INTRODUCTION: Etizolam [4-(2-chlorophenyl)-2-ethyl-9-methyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine] is a benzodiazepine analogue. The benzene ring is replaced with a thiophene ring making the drug a ‘thienodiazepine’. This poster reviews four post-mortem cases, submitted to Imperial College Toxicology Unit, in which etizolam was detected in the post-mortem, femoral blood. RESULTS: The findings are summarised in the tables below. Medicinal use: • Widely available in Japan (Depas, Sedekopan ) and India (Etilaam, Etizola). CASE 1: CASE 2: • Adult daily doses range from 0.5 – 3.0 milligrams. • 52 year old female • 47 year old male • Used in short-term treatment of insomnia or anxiety. 1 • Found dead in church yard surrounded by empty • History of alcohol abuse and depression 2 • Acts as a full agonist at the benzodiazepine receptor. medication packets • Has potent hypnotic properties comparable with other short-acting • Found dead in bed surrounded by empty medication benzodiazepines. Drug Blood Concentration packets. • (Etizolam has anxiolytic effects six times greater than diazepam). 3 ETIZOLAM 0.09 µg/ml Drug Blood Concentration 4 • Thought to have reduced tolerance/dependence than classical benzodiazepines. Diphenhydramine High therapeutic ETIZOLAM 0.46 µg/ml FIGURE 1: Structure of Etizolam • However, long-term use may produce similar side-effects to benzodiazepines: Venlafaxine 7.10 µg/ml 5 Amitriptyline High therapeutic addiction, hostile behaviour, memory loss and severe withdrawal. Mirtazapine 1.41 µg/ml Illicit use: Ethanol <10 mg/dL Citalopram 0.79 µg/ml • Etizolam is not licenced as a medicine in the UK.