Appendix 1 Cross-Reference of Research, Generic and Trade Names of Benzodiazepines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guaiana, G., Barbui, C., Caldwell, DM, Davies, SJC, Furukawa, TA
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Explore Bristol Research Guaiana, G., Barbui, C., Caldwell, D. M., Davies, S. J. C., Furukawa, T. A., Imai, H., ... Cipriani, A. (2017). Antidepressants, benzodiazepines and azapirones for panic disorder in adults: a network meta-analysis. Cochrane Database of Systematic Reviews, 2017(7), [CD012729]. https://doi.org/10.1002/14651858.CD012729 Publisher's PDF, also known as Version of record Link to published version (if available): 10.1002/14651858.CD012729 Link to publication record in Explore Bristol Research PDF-document This is the final published version of the article (version of record). It first appeared online via Cochrane Library at https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012729/full . Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/pure/about/ebr-terms Cochrane Database of Systematic Reviews Antidepressants, benzodiazepines and azapirones for panic disorder in adults: a network meta-analysis (Protocol) Guaiana G, Barbui C, Caldwell DM, Davies SJC, Furukawa TA, Imai H, Koesters M, Tajika A, Bighelli I, Pompoli A, Cipriani A Guaiana G, Barbui C, Caldwell DM, Davies SJC, Furukawa TA, Imai H, Koesters M, Tajika A, Bighelli I, Pompoli A, Cipriani A. Antidepressants, benzodiazepines and azapirones for panic disorder in adults: a network meta-analysis. Cochrane Database of Systematic Reviews 2017, Issue 7. -

Understanding Benzodiazephine Use, Abuse, and Detection
Siemens Healthcare Diagnostics, the leading clinical diagnostics company, is committed to providing clinicians with the vital information they need for the accurate diagnosis, treatment and monitoring of patients. Our comprehensive portfolio of performance-driven systems, unmatched menu offering and IT solutions, in conjunction with highly responsive service, is designed to streamline workflow, enhance operational efficiency and support improved patient care. Syva, EMIT, EMIT II, EMIT d.a.u., and all associated marks are trademarks of General Siemens Healthcare Diagnostics Inc. All Drugs other trademarks and brands are the Global Division property of their respective owners. of Abuse Siemens Healthcare Product availability may vary from Diagnostics Inc. country to country and is subject 1717 Deerfield Road to varying regulatory requirements. Deerfield, IL 60015-0778 Please contact your local USA representative for availability. www.siemens.com/diagnostics Siemens Global Headquarters Global Siemens Healthcare Headquarters Siemens AG Understanding Wittelsbacherplatz 2 Siemens AG 80333 Muenchen Healthcare Sector Germany Henkestrasse 127 Benzodiazephine Use, 91052 Erlangen Germany Abuse, and Detection Telephone: +49 9131 84 - 0 www.siemens.com/healthcare www.usa.siemens.com/diagnostics Answers for life. Order No. A91DX-0701526-UC1-4A00 | Printed in USA | © 2009 Siemens Healthcare Diagnostics Inc. Syva has been R1 R2 a leading developer N and manufacturer of AB R3 X N drugs-of-abuse tests R4 for more than 30 years. R2 C Now part of Siemens Healthcare ® Diagnostics, Syva boasts a long and Benzodiazepines have as their basic chemical structure successful track record in drugs-of-abuse a benzene ring fused to a seven-membered diazepine ring. testing, and leads the industry in the All important benzodiazepines contain a 5-aryl substituent ring (ring C) and a 1,4–diazepine ring. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Information to Users
Confirmation of urinary benzodiazepines by gas chromatography/mass spectrometry Item Type text; Thesis-Reproduction (electronic) Authors West, Robert E., 1952- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 06/10/2021 02:51:08 Link to Item http://hdl.handle.net/10150/277228 INFORMATION TO USERS The most advanced technology has been used to photo graph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are re produced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. -
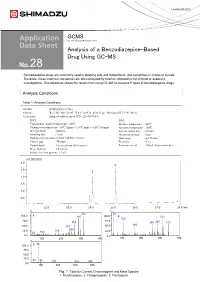
Analysis of a Benzodiazepine-Based Drug Using GC-MS 28
LAAN-E-MS-E028 GCMS Gas Chromatograph Mass Spectrometer Analysis of a Benzodiazepine-Based Drug Using GC-MS 28 Benzodiazepine drugs are commonly used in sleeping aids and tranquilizers, and sometimes in crimes or suicide. Therefore, these chemical substances are often analyzed by forensic laboratories for criminal or academic investigations. This datasheet shows the results from using GC-MS to measure 9 types of benzodiazepine drugs. Analysis Conditions Table 1: Analysis Conditions GC-MS :GCMS-QP2010 Ultra Column : Rxi®-5Sil MS (30 mL. X 0.25 mmI.D., df=0.25 µm, Shimadzu GLC P/N:13623) Glass insert :Silanized splitless insert (P/N: 221-48876-03) [GC] [MS] Vaporization chamber temperature : 260℃ Interface temperature : 280℃ Column oven temperature : 60℃ (2min) -> (10℃/min) -> 320℃ (10min) Ion source temperature : 200℃ Injection mode : Splitless Solvent elution time : 2.0 min Sampling time : 1 min Measurement mode : Scan High pressure injection method: 250 kPa (1.5 min) Mass range : m/z 35-600 Carrier gas : Helium Event time : 0.3 sec Control mode :Linear velocity (45.6 cm/sec) Emission current : 150 µA (high sensitivity) Purge flow rate :3.0 ml/min Sample injection quantity :1.0 µL (x1,000,000) 3.0 3 2.5 2 2.0 1.5 1.0 1 0.5 22.0 23.0 24.0 25.0 26.0 27.0 28.0 min % % 312 55 100.0 1 285 100.0 2 313 109 75.0 75.0 266 259287 342 166 50.0 238 50.0 248 25.0 183 25.0 63 109 0.0 0.0 100 200 300 400 100 200 300 400 % 86 100.0 3 75.0 50.0 25.0 58 99 183 315 387 0.0 100 200 300 400 Fig. -

Proposed Regulation of the State Board of Pharmacy
PROPOSED REGULATION OF THE STATE BOARD OF PHARMACY LCB File No. R133-14 Workshop July 24, 2014 NAC 453.540 Schedule IV. (NRS 453.146, 639.070) 1. Schedule IV consists of the drugs and other substances listed in this section, by whatever official, common, usual, chemical or trade name designated. 2. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture or preparation containing any of the following narcotic drugs, including, without limitation, their salts, calculated as the free anhydrous base of alkaloid, is hereby enumerated on schedule IV, in quantities: (a) Not more than 1 milligram of difenoxin and not less than 25 micrograms of atropine sulfate per dosage unit; or (b) Dextropropoxyphene (alpha-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2-propionoxy- butane). 3. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture or preparation which contains any quantity of the following substances, including, without limitation, their salts, isomers and salts of isomers, is hereby enumerated on schedule IV, whenever the existence of such salts, isomers and salts of isomers is possible within the specific chemical designation: Alprazolam; Barbital; Bromazepam; Butorphanol; Camazepam; Carisoprodol; Chloral betaine; Chloral hydrate; Chlordiazepoxide; Clobazam; Clonazepam; Clorazepate; Clotiazepam; Cloxazolam; Delorazepam; Diazepam; Dichloralphenazone; Estazolam; Ethchlorvynol; Ethyl loflazepate; Fludiazepam; Flunitrazepam; --1-- Agency Draft of Proposed Regulation R133-14 Flurazepam; Halazepam; Haloxazolam; Ketazolam; Loprazolam; Lorazepam; Lormetazepam; Mebutamate; Medazepam; Meprobamate; Methohexital; Methylphenobarbital (mephobarbital); Midazolam; Nimetazepam; Nitrazepam; Nordiazepam; Oxazepam; Oxazolam; Paraldehyde; Petrichloral; Phenobarbital; Pinazepam; Prazepam; Quazepam; Tramadol (2-((dimethylamino)methyl)-1-(3-methoxyphenyl)cyclohexanol) Temazepam; Tetrazepam; Triazolam; Zaleplon; Zolpidem; or Zopiclone. -

Recommended Methods for the Identification and Analysis of Fentanyl and Its Analogues in Biological Specimens
Recommended methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2017 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/53 Original language: English © United Nations, November 2017. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. Barry Logan, Center for Forensic Science Research and Education, at the Fredric Rieders Family Founda- tion and NMS Labs, United States; Amanda L.A. -

DEMAND REDUCTION a Glossary of Terms
UNITED NATIONS PUBLICATION Sales No. E.00.XI.9 ISBN: 92-1-148129-5 ACKNOWLEDGEMENTS This document was prepared by the: United Nations International Drug Control Programme (UNDCP), Vienna, Austria, in consultation with the Commonwealth of Health and Aged Care, Australia, and the informal international reference group. ii Contents Page Foreword . xi Demand reduction: A glossary of terms . 1 Abstinence . 1 Abuse . 1 Abuse liability . 2 Action research . 2 Addiction, addict . 2 Administration (method of) . 3 Adverse drug reaction . 4 Advice services . 4 Advocacy . 4 Agonist . 4 AIDS . 5 Al-Anon . 5 Alcohol . 5 Alcoholics Anonymous (AA) . 6 Alternatives to drug use . 6 Amfetamine . 6 Amotivational syndrome . 6 Amphetamine . 6 Amyl nitrate . 8 Analgesic . 8 iii Page Antagonist . 8 Anti-anxiety drug . 8 Antidepressant . 8 Backloading . 9 Bad trip . 9 Barbiturate . 9 Benzodiazepine . 10 Blood-borne virus . 10 Brief intervention . 11 Buprenorphine . 11 Caffeine . 12 Cannabis . 12 Chasing . 13 Cocaine . 13 Coca leaves . 14 Coca paste . 14 Cold turkey . 14 Community empowerment . 15 Co-morbidity . 15 Comprehensive Multidisciplinary Outline of Future Activities in Drug Abuse Control (CMO) . 15 Controlled substance . 15 Counselling and psychotherapy . 16 Court diversion . 16 Crash . 16 Cross-dependence . 17 Cross-tolerance . 17 Custody diversion . 17 Dance drug . 18 Decriminalization or depenalization . 18 Demand . 18 iv Page Demand reduction . 19 Dependence, dependence syndrome . 19 Dependence liability . 20 Depressant . 20 Designer drug . 20 Detoxification . 20 Diacetylmorphine/Diamorphine . 21 Diuretic . 21 Drug . 21 Drug abuse . 22 Drug abuse-related harm . 22 Drug abuse-related problem . 22 Drug policy . 23 Drug seeking . 23 Drug substitution . 23 Drug testing . 24 Drug use . -

Pharmacological Properties of GABAA- Receptors Containing Gamma1
Molecular Pharmacology Fast Forward. Published on November 4, 2005 as DOI: 10.1124/mol.105.017236 Molecular PharmacologyThis article hasFast not Forward.been copyedited Published and formatted. on The November final version 7, may 2005 differ as from doi:10.1124/mol.105.017236 this version. MOLPHARM/2005/017236 Pharmacological properties of GABAA- receptors containing gamma1- subunits Khom S.1, Baburin I.1, Timin EN, Hohaus A., Sieghart W., Hering S. Downloaded from Department of Pharmacology and Toxicology, University of Vienna Center of Brain Research , Medical University of Vienna, Division of Biochemistry and molpharm.aspetjournals.org Molecular Biology at ASPET Journals on September 27, 2021 1 Copyright 2005 by the American Society for Pharmacology and Experimental Therapeutics. Molecular Pharmacology Fast Forward. Published on November 4, 2005 as DOI: 10.1124/mol.105.017236 This article has not been copyedited and formatted. The final version may differ from this version. MOLPHARM/2005/017236 Running Title: GABAA- receptors containing gamma1- subunits Corresponding author: Steffen Hering Department of Pharmacology and Toxicology University of Vienna Althanstrasse 14 Downloaded from A-1090 Vienna Telephone number: +43-1-4277-55301 Fax number: +43-1-4277-9553 molpharm.aspetjournals.org [email protected] Text pages: 29 at ASPET Journals on September 27, 2021 Tables: 2 Figures: 7 References: 26 Abstract: 236 words Introduction:575 words Discussion:1383 words 2 Molecular Pharmacology Fast Forward. Published on November 4, 2005 as DOI: 10.1124/mol.105.017236 This article has not been copyedited and formatted. The final version may differ from this version. MOLPHARM/2005/017236 Abstract GABAA receptors composed of α1, β2, γ1-subunits are expressed in only a few areas of the brain and thus represent interesting drug targets. -

Meta-Analysis of Benzodiazepine Use in the Treatment of Insomnia
Meta-analysis of benzodiazepine use in the treatment of insomnia Review Anne M. Holbrook, Renée Crowther, Ann Lotter, Chiachen Cheng, Derek King Synthèse Abstract From the Centre for Evaluation of Medicines, Objective: To systematically review the benefits and risks associated with the use St. Joseph’s Hospital of benzodiazepines to treat insomnia in adults. and McMaster University, Data sources: MEDLINE and the Cochrane Controlled Trials Registry were Hamilton, Ont. searched for English-language articles published from 1966 to December 1998 that described randomized controlled trials of benzodiazepines for the treatment This article has been peer reviewed. of insomnia. Key words included “benzodiazepines” (exploded), “randomized controlled trial” and “insomnia.” Bibliographies of relevant articles were re- CMAJ 2000;162(2):225-33 viewed for additional studies and manufacturers of benzodiazepines were asked to submit additional randomized controlled trial reports not in the literature. ß An overview of the diagnosis and management of insomnia in clinical Study selection: Articles were considered for the meta-analysis if they were ran- practice appears on page 216. domized controlled trials involving patients with insomnia and compared a ben- zodiazepine with placebo or another active agent. Of the 89 trials originally identified, 45 met our criteria, representing a total of 2672 patients. January 25, 2000 Data extraction: Data were extracted regarding the participants, the setting, details Table of Contents of the intervention, the outcomes (including adverse effects) and the method- ologic quality of the studies. Data synthesis: The meta-analyses of sleep records indicated that, when compared with placebo, benzodiazepines decreased sleep latency by 4.2 minutes (non- significant; 95% confidence interval [CI] –0.7 to 9.2) and significantly increased total sleep duration by 61.8 minutes (95% CI 37.4 to 86.2). -

Comparative Efficacy and Acceptability of Pharmacological Treatments for Insomnia in Adults: a Systematic Review and Network Meta-Analysis (Protocol)
Cochrane Database of Systematic Reviews Comparative efficacy and acceptability of pharmacological treatments for insomnia in adults: a systematic review and network meta-analysis (Protocol) De Crescenzo F, Foti F, Ciabattini M, Del Giovane C, Watanabe N, Sañé Schepisi M, Quested DJ, Cipriani A, Barbui C, Amato L De Crescenzo F, Foti F, Ciabattini M, Del Giovane C, Watanabe N, Sañé Schepisi M, Quested DJ, Cipriani A, Barbui C, Amato L. Comparative efficacy and acceptability of pharmacological treatments for insomnia in adults: a systematic review and network meta-anal- ysis. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No.: CD012364. DOI: 10.1002/14651858.CD012364. www.cochranelibrary.com Comparative efficacy and acceptability of pharmacological treatments for insomnia in adults: a systematic review and network meta- analysis (Protocol) Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 BACKGROUND .................................... 1 OBJECTIVES ..................................... 4 METHODS ...................................... 4 Figure1. ..................................... 5 ACKNOWLEDGEMENTS . 9 REFERENCES ..................................... 10 APPENDICES ..................................... 14 WHAT’SNEW..................................... 18 CONTRIBUTIONSOFAUTHORS . 18 DECLARATIONSOFINTEREST . 18 Comparative efficacy and acceptability of pharmacological treatments for -

Effects of Brotizolam, a New Thieno-Triazolo-Diazepine Derivative, on the Central Nervous System
In the present study, its actions on the central nervous system were investigated. Effects of Brotizolam, a New Thieno-Triazolo-Diazepine Derivative, on the Central Nervous System Kenjiro KIMISHIMA, Kyoko TANABE, Yukako KINOSHITA, Kooji TOKUYOSHI, Daisuke HOURI and Tatsuo KOBAYASHI Department of Pharmacology, Tottori University School of Medicine, Yonago 683, Japan Accepted August 24, 1984 Abstract-The effects of brotizolam, a new thieno-triazolo-diazepine derivative, on the central nervous system were analyzed in mice, rats and rabbits. Diazepam, estazolam and triazolam were used as control drugs. Brotizolam inhibited spon taneous motor activities; performances in the rotarod test, staircase test, and maximal electroshock seizure test; and pentetrazol or bemegride-induced convulsion. Moreover, catalepsy inducing action and potentiating effect on sleep elicited by pentobarbital or ethanol were observed. Following intraperitoneal or oral admin istration of brotizolam to rabbits with chronically implanted electrodes , the electro encephalographic profile in spontaneous EEG was characterized by slow waves with high amplitudes in the neocortex. The arousal responses by stimulation of the midbrain reticular formation and posterior hypothalamus were slightly inhibited, but the recruiting responses induced by stimulation of the diffuse thalamic projecting system were not inhibited, and seizure discharges induced by stimulation of the dorsal hippocampus were inhibited markedly. When motor activities and pente trazol-induced convulsions were observed as indices of tolerance for brotizolam, tolerance was not developed by repeated administration of brotizolam up to 14 days. These results suggested that brotizolam, a new thieno-triazolo-diazepine derivative, is judged to be a safer and stronger sleep inducer than diazepam and estazolam . Since the pioneering paper by Randall et et al.