Use and Abuse of Benzodiazepines*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Methylphenidate Hydrochloride
Application for Inclusion to the 22nd Expert Committee on the Selection and Use of Essential Medicines: METHYLPHENIDATE HYDROCHLORIDE December 7, 2018 Submitted by: Patricia Moscibrodzki, M.P.H., and Craig L. Katz, M.D. The Icahn School of Medicine at Mount Sinai Graduate Program in Public Health New York NY, United States Contact: [email protected] TABLE OF CONTENTS Page 3 Summary Statement Page 4 Focal Point Person in WHO Page 5 Name of Organizations Consulted Page 6 International Nonproprietary Name Page 7 Formulations Proposed for Inclusion Page 8 International Availability Page 10 Listing Requested Page 11 Public Health Relevance Page 13 Treatment Details Page 19 Comparative Effectiveness Page 29 Comparative Safety Page 41 Comparative Cost and Cost-Effectiveness Page 45 Regulatory Status Page 48 Pharmacoepial Standards Page 49 Text for the WHO Model Formulary Page 52 References Page 61 Appendix – Letters of Support 2 1. Summary Statement of the Proposal for Inclusion of Methylphenidate Methylphenidate (MPH), a central nervous system (CNS) stimulant, of the phenethylamine class, is proposed for inclusion in the WHO Model List of Essential Medications (EML) & the Model List of Essential Medications for Children (EMLc) for treatment of Attention-Deficit/Hyperactivity Disorder (ADHD) under ICD-11, 6C9Z mental, behavioral or neurodevelopmental disorder, disruptive behavior or dissocial disorders. To date, the list of essential medications does not include stimulants, which play a critical role in the treatment of psychotic disorders. Methylphenidate is proposed for inclusion on the complimentary list for both children and adults. This application provides a systematic review of the use, efficacy, safety, availability, and cost-effectiveness of methylphenidate compared with other stimulant (first-line) and non-stimulant (second-line) medications. -

Substance Abuse and Dependence
9 Substance Abuse and Dependence CHAPTER CHAPTER OUTLINE CLASSIFICATION OF SUBSTANCE-RELATED THEORETICAL PERSPECTIVES 310–316 Residential Approaches DISORDERS 291–296 Biological Perspectives Psychodynamic Approaches Substance Abuse and Dependence Learning Perspectives Behavioral Approaches Addiction and Other Forms of Compulsive Cognitive Perspectives Relapse-Prevention Training Behavior Psychodynamic Perspectives SUMMING UP 325–326 Racial and Ethnic Differences in Substance Sociocultural Perspectives Use Disorders TREATMENT OF SUBSTANCE ABUSE Pathways to Drug Dependence AND DEPENDENCE 316–325 DRUGS OF ABUSE 296–310 Biological Approaches Depressants Culturally Sensitive Treatment Stimulants of Alcoholism Hallucinogens Nonprofessional Support Groups TRUTH or FICTION T❑ F❑ Heroin accounts for more deaths “Nothing and Nobody Comes Before than any other drug. (p. 291) T❑ F❑ You cannot be psychologically My Coke” dependent on a drug without also being She had just caught me with cocaine again after I had managed to convince her that physically dependent on it. (p. 295) I hadn’t used in over a month. Of course I had been tooting (snorting) almost every T❑ F❑ More teenagers and young adults die day, but I had managed to cover my tracks a little better than usual. So she said to from alcohol-related motor vehicle accidents me that I was going to have to make a choice—either cocaine or her. Before she than from any other cause. (p. 297) finished the sentence, I knew what was coming, so I told her to think carefully about what she was going to say. It was clear to me that there wasn’t a choice. I love my T❑ F❑ It is safe to let someone who has wife, but I’m not going to choose anything over cocaine. -

Understanding Benzodiazephine Use, Abuse, and Detection
Siemens Healthcare Diagnostics, the leading clinical diagnostics company, is committed to providing clinicians with the vital information they need for the accurate diagnosis, treatment and monitoring of patients. Our comprehensive portfolio of performance-driven systems, unmatched menu offering and IT solutions, in conjunction with highly responsive service, is designed to streamline workflow, enhance operational efficiency and support improved patient care. Syva, EMIT, EMIT II, EMIT d.a.u., and all associated marks are trademarks of General Siemens Healthcare Diagnostics Inc. All Drugs other trademarks and brands are the Global Division property of their respective owners. of Abuse Siemens Healthcare Product availability may vary from Diagnostics Inc. country to country and is subject 1717 Deerfield Road to varying regulatory requirements. Deerfield, IL 60015-0778 Please contact your local USA representative for availability. www.siemens.com/diagnostics Siemens Global Headquarters Global Siemens Healthcare Headquarters Siemens AG Understanding Wittelsbacherplatz 2 Siemens AG 80333 Muenchen Healthcare Sector Germany Henkestrasse 127 Benzodiazephine Use, 91052 Erlangen Germany Abuse, and Detection Telephone: +49 9131 84 - 0 www.siemens.com/healthcare www.usa.siemens.com/diagnostics Answers for life. Order No. A91DX-0701526-UC1-4A00 | Printed in USA | © 2009 Siemens Healthcare Diagnostics Inc. Syva has been R1 R2 a leading developer N and manufacturer of AB R3 X N drugs-of-abuse tests R4 for more than 30 years. R2 C Now part of Siemens Healthcare ® Diagnostics, Syva boasts a long and Benzodiazepines have as their basic chemical structure successful track record in drugs-of-abuse a benzene ring fused to a seven-membered diazepine ring. testing, and leads the industry in the All important benzodiazepines contain a 5-aryl substituent ring (ring C) and a 1,4–diazepine ring. -
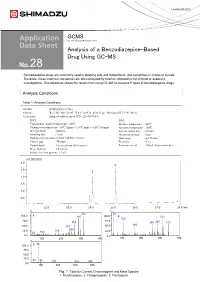
Analysis of a Benzodiazepine-Based Drug Using GC-MS 28
LAAN-E-MS-E028 GCMS Gas Chromatograph Mass Spectrometer Analysis of a Benzodiazepine-Based Drug Using GC-MS 28 Benzodiazepine drugs are commonly used in sleeping aids and tranquilizers, and sometimes in crimes or suicide. Therefore, these chemical substances are often analyzed by forensic laboratories for criminal or academic investigations. This datasheet shows the results from using GC-MS to measure 9 types of benzodiazepine drugs. Analysis Conditions Table 1: Analysis Conditions GC-MS :GCMS-QP2010 Ultra Column : Rxi®-5Sil MS (30 mL. X 0.25 mmI.D., df=0.25 µm, Shimadzu GLC P/N:13623) Glass insert :Silanized splitless insert (P/N: 221-48876-03) [GC] [MS] Vaporization chamber temperature : 260℃ Interface temperature : 280℃ Column oven temperature : 60℃ (2min) -> (10℃/min) -> 320℃ (10min) Ion source temperature : 200℃ Injection mode : Splitless Solvent elution time : 2.0 min Sampling time : 1 min Measurement mode : Scan High pressure injection method: 250 kPa (1.5 min) Mass range : m/z 35-600 Carrier gas : Helium Event time : 0.3 sec Control mode :Linear velocity (45.6 cm/sec) Emission current : 150 µA (high sensitivity) Purge flow rate :3.0 ml/min Sample injection quantity :1.0 µL (x1,000,000) 3.0 3 2.5 2 2.0 1.5 1.0 1 0.5 22.0 23.0 24.0 25.0 26.0 27.0 28.0 min % % 312 55 100.0 1 285 100.0 2 313 109 75.0 75.0 266 259287 342 166 50.0 238 50.0 248 25.0 183 25.0 63 109 0.0 0.0 100 200 300 400 100 200 300 400 % 86 100.0 3 75.0 50.0 25.0 58 99 183 315 387 0.0 100 200 300 400 Fig. -

XANAX® Alprazolam Tablets, USP
XANAX® alprazolam tablets, USP CIV WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death [see Warnings, Drug Interactions]. Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation. DESCRIPTION XANAX Tablets contain alprazolam which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds. The chemical name of alprazolam is 8-Chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-α] [1,4] benzodiazepine. The structural formula is represented to the right: Alprazolam is a white crystalline powder, which is soluble in methanol or ethanol but which has no appreciable solubility in water at physiological pH. Each XANAX Tablet, for oral administration, contains 0.25, 0.5, 1 or 2 mg of alprazolam. XANAX Tablets, 2 mg, are multi-scored and may be divided as shown below: 1 Reference ID: 4029640 Inactive ingredients: Cellulose, corn starch, docusate sodium, lactose, magnesium stearate, silicon dioxide and sodium benzoate. In addition, the 0.5 mg tablet contains FD&C Yellow No. 6 and the 1 mg tablet contains FD&C Blue No. 2. CLINICAL PHARMACOLOGY Pharmacodynamics CNS agents of the 1,4 benzodiazepine class presumably exert their effects by binding at stereo specific receptors at several sites within the central nervous system. Their exact mechanism of action is unknown. Clinically, all benzodiazepines cause a dose-related central nervous system depressant activity varying from mild impairment of task performance to hypnosis. -

Analytical Methods for Determination of Benzodiazepines. a Short Review
Cent. Eur. J. Chem. • 12(10) • 2014 • 994-1007 DOI: 10.2478/s11532-014-0551-1 Central European Journal of Chemistry Analytical methods for determination of benzodiazepines. A short review Review Article Paulina Szatkowska1, Marcin Koba1*, Piotr Kośliński1, Jacek Wandas1, Tomasz Bączek2,3 1Department of Toxicology, Faculty of Pharmacy, Collegium Medicum of Nicolaus Copernicus University, 85-089 Bydgoszcz, Poland 2Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Medical University of Gdańsk, 80-416 Gdańsk, Poland 3Institute of Health Sciences, Division of Human Anatomy and Physiology, Pomeranian University of Słupsk, 76-200 Słupsk, Poland Received 16 July 2013; Accepted 6 February 2014 Abstract: Benzodiazepines (BDZs) are generally commonly used as anxiolytic and/or hypnotic drugs as a ligand of the GABAA-benzodiazepine receptor. Moreover, some of benzodiazepines are widely used as an anti-depressive and sedative drugs, and also as anti-epileptic drugs and in some cases can be useful as an adjunct treatment in refractory epilepsies or anti-alcoholic therapy. High-performance liquid chromatography (HPLC) methods, thin-layer chromatography (TLC) methods, gas chromatography (GC) methods, capillary electrophoresis (CE) methods and some of spectrophotometric and spectrofluorometric methods were developed and have been extensively applied to the analysis of number of benzodiazepine derivative drugs (BDZs) providing reliable and accurate results. The available chemical methods for the determination of BDZs in biological materials and pharmaceutical formulations are reviewed in this work. Keywords: Analytical methods • Benzodiazepines • Drugs analysis • Pharmaceutical formulations © Versita Sp. z o.o. 1. Introduction and long). For this reason, an application of these drugs became broader allowing their utility to a larger extent, Benzodiazepines have been first introduced into medical and at the same time, problems related to drug abuse practice in the 60s of the last century. -

Recommended Methods for the Identification and Analysis of Fentanyl and Its Analogues in Biological Specimens
Recommended methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2017 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/53 Original language: English © United Nations, November 2017. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. Barry Logan, Center for Forensic Science Research and Education, at the Fredric Rieders Family Founda- tion and NMS Labs, United States; Amanda L.A. -

Is Cannabis Addictive?
Is cannabis addictive? CANNABIS EVIDENCE BRIEF BRIEFS AVAILABLE IN THIS SERIES: ` Is cannabis safe to use? Facts for youth aged 13–17 years. ` Is cannabis safe to use? Facts for young adults aged 18–25 years. ` Does cannabis use increase the risk of developing psychosis or schizophrenia? ` Is cannabis safe during preconception, pregnancy and breastfeeding? ` Is cannabis addictive? PURPOSE: This document provides key messages and information about addiction to cannabis in adults as well as youth between 16 and 18 years old. It is intended to provide source material for public education and awareness activities undertaken by medical and public health professionals, parents, educators and other adult influencers. Information and key messages can be re-purposed as appropriate into materials, including videos, brochures, etc. © Her Majesty the Queen in Right of Canada, as represented by the Minister of Health, 2018 Publication date: August 2018 This document may be reproduced in whole or in part for non-commercial purposes, without charge or further permission, provided that it is reproduced for public education purposes and that due diligence is exercised to ensure accuracy of the materials reproduced. Cat.: H14-264/3-2018E-PDF ISBN: 978-0-660-27409-6 Pub.: 180232 Key messages ` Cannabis is addictive, though not everyone who uses it will develop an addiction.1, 2 ` If you use cannabis regularly (daily or almost daily) and over a long time (several months or years), you may find that you want to use it all the time (craving) and become unable to stop on your own.3, 4 ` Stopping cannabis use after prolonged use can produce cannabis withdrawal symptoms.5 ` Know that there are ways to change this and people who can help you. -

Hsrs Alcohol and Other Drug Abuse Module
WORKER ID (Field 1) OPTIONAL DEFINITION: The primary worker assigned to the client, or the person designated by the agency as having overall responsibility for the client or case. This is the person who will get information back about the client if any is requested. You may use a provider ID if you have delegated overall responsibility to a provider and you want them to get back all information about this client. PURPOSE: For local use to connect reports to specific case managers. MA NUMBER (Field 2) REQUIRED IF MEDICAL ASSISTANCE RECIPIENT CODES: Enter the client’s 10 digit MA Number. PURPOSE: For comparison with other databases (Medical Assistance; DWD employment data; Crime Information Bureau, etc.) CLIENT ID (Field 3) REQUIRED, COMPUTER GENERATED DEFINITION: An identifier that is computer generated for each individual reported on HSRS. Full legal name, birthdate, and sex are used to produce a 14 character ID which bears no resemblance to the client’s name. ENTER: May be left blank if name, birthdate, and sex are reported. OR Enter the 14 character HSRS client identification number. The ID will be generated and returned to you on the terminal screen. Copy it down or print the screen. Once the ID number is generated, use it on all future input. PURPOSE: To maintain client confidentiality while allowing reports to be produced on individual clients for audit purposes; to produce reports on multiple services to the same individual; to produce client number listings for recidivist clients. AODA - 1 NAME - LAST, FIRST, MIDDLE, SUFFIX (Fields 4a-d) REQUIRED TO GENERATE ID (THEN OPTIONAL) DEFINITION: The full legal name of the client. -

Can Tobacco Dependence Provide Insights Into Other Drug Addictions? Joseph R
DiFranza BMC Psychiatry (2016) 16:365 DOI 10.1186/s12888-016-1074-4 DEBATE Open Access Can tobacco dependence provide insights into other drug addictions? Joseph R. DiFranza Abstract Within the field of addiction research, individuals tend to operate within silos of knowledge focused on specific drug classes. The discovery that tobacco dependence develops in a progression of stages and that the latency to the onset of withdrawal symptoms after the last use of tobacco changes over time have provided insights into how tobacco dependence develops that might be applied to the study of other drugs. As physical dependence on tobacco develops, it progresses through previously unrecognized clinical stages of wanting, craving and needing. The latency to withdrawal is a measure of the asymptomatic phase of withdrawal, extending from the last use of tobacco to the emergence of withdrawal symptoms. Symptomatic withdrawal is characterized by a wanting phase, a craving phase, and a needing phase. The intensity of the desire to smoke that is triggered by withdrawal correlates with brain activity in addiction circuits. With repeated tobacco use, the latency to withdrawal shrinks from as long as several weeks to as short as several minutes. The shortening of the asymptomatic phase of withdrawal drives an escalation of smoking, first in terms of the number of smoking days/ month until daily smoking commences, then in terms of cigarettes smoked/day. The discoveries of the stages of physical dependence and the latency to withdrawal raises the question, does physical dependence develop in stages with other drugs? Is the latency to withdrawal for other substances measured in weeks at the onset of dependence? Does it shorten over time? The research methods that uncovered how tobacco dependence emerges might be fruitfully applied to the investigation of other addictions. -

Patient-Focused Drug Development Meeting on Opioid Use Disorder
Patient-Focused Drug Development Meeting on Opioid Use Disorder April 17, 2018 FDA will be streaming a live audio recording of the meeting with the presentation slides, which is open to the public at: https://collaboration.fda.gov/pfdd041718/. The audio recording and presentation slides, along with a meeting transcript and summary report, will also be made publicly available after the meeting. Because of the sensitive nature of the meeting topic, and the importance of gathering candid, meaningful input from individuals who have come forward to speak about living with opioid use disorder, no other audio recording, video recording, and/or photography will be allowed at this Patient-Focused Drug Development meeting. FDA is asking for your cooperation and strongly requests that you respect the privacy of all attendees. #PFDD Wi-Fi Network: FDA-Public Password: publicaccess Welcome Sara Eggers, PhD Office of Strategic Programs Center for Drug Evaluation and Research April 17, 2018 U.S. Food and Drug Administration Agenda • Opening Remarks • Setting the context – Overview of Opioid Use Disorder – Road from PFDD Meetings to Clinical Trial Endpoints – Overview of Discussion Format • Discussion Topic 1 • Lunch • Discussion Topic 2 (with a short break) • Open Public Comment • Closing Remarks 3 3 No Recording or Photography • FDA is streaming a live audio recording of the meeting with the presentation slides, which is open to the public – Access the live stream: https://collaboration.fda.gov/pfdd041718/. – The audio recording and presentation slides, along with a meeting transcript and summary report, will also be made publicly available after the meeting. • Because of the sensitive nature of the meeting topic, and the importance of gathering candid, meaningful input from individuals who have come forward to speak about living with opioid use disorder, no other audio recording, video recording, and/or photography will be allowed at this Patient-Focused Drug Development meeting. -

124.210 Schedule IV — Substances Included. 1
1 CONTROLLED SUBSTANCES, §124.210 124.210 Schedule IV — substances included. 1. Schedule IV shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. 2. Narcotic drugs. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation containing any of the following narcotic drugs, or their salts calculated as the free anhydrous base or alkaloid, in limited quantities as set forth below: a. Not more than one milligram of difenoxin and not less than twenty-five micrograms of atropine sulfate per dosage unit. b. Dextropropoxyphene (alpha-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2- propionoxybutane). c. 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol, its salts, optical and geometric isomers and salts of these isomers (including tramadol). 3. Depressants. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances, including its salts, isomers, and salts of isomers whenever the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation: a. Alprazolam. b. Barbital. c. Bromazepam. d. Camazepam. e. Carisoprodol. f. Chloral betaine. g. Chloral hydrate. h. Chlordiazepoxide. i. Clobazam. j. Clonazepam. k. Clorazepate. l. Clotiazepam. m. Cloxazolam. n. Delorazepam. o. Diazepam. p. Dichloralphenazone. q. Estazolam. r. Ethchlorvynol. s. Ethinamate. t. Ethyl Loflazepate. u. Fludiazepam. v. Flunitrazepam. w. Flurazepam. x. Halazepam. y. Haloxazolam. z. Ketazolam. aa. Loprazolam. ab. Lorazepam. ac. Lormetazepam. ad. Mebutamate. ae. Medazepam. af. Meprobamate. ag. Methohexital. ah. Methylphenobarbital (mephobarbital).