The Phenotypic Markers of CD4+CD25+ T Regulatory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

Chronic Myeloid Leukemia: Mechanisms of Blastic Transformation
Chronic myeloid leukemia: mechanisms of blastic transformation Danilo Perrotti, … , John Goldman, Tomasz Skorski J Clin Invest. 2010;120(7):2254-2264. https://doi.org/10.1172/JCI41246. Science in Medicine The BCR-ABL1 oncoprotein transforms pluripotent HSCs and initiates chronic myeloid leukemia (CML). Patients with early phase (also known as chronic phase [CP]) disease usually respond to treatment with ABL tyrosine kinase inhibitors (TKIs), although some patients who respond initially later become resistant. In most patients, TKIs reduce the leukemia cell load substantially, but the cells from which the leukemia cells are derived during CP (so-called leukemia stem cells [LSCs]) are intrinsically insensitive to TKIs and survive long term. LSCs or their progeny can acquire additional genetic and/or epigenetic changes that cause the leukemia to transform from CP to a more advanced phase, which has been subclassified as either accelerated phase or blastic phase disease. The latter responds poorly to treatment and is usually fatal. Here, we discuss what is known about the molecular mechanisms leading to blastic transformation of CML and propose some novel therapeutic approaches. Find the latest version: https://jci.me/41246/pdf Science in medicine Chronic myeloid leukemia: mechanisms of blastic transformation Danilo Perrotti,1 Catriona Jamieson,2 John Goldman,3 and Tomasz Skorski4 1Department of Molecular Virology, Immunology and Medical Genetics and Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio, USA. 2Division of Hematology-Oncology, Department of Internal Medicine, University of California at San Diego, La Jolla, California, USA. 3Department of Haematology, Imperial College at Hammersmith Hospital, London, United Kingdom. 4Department of Microbiology and Immunology, Temple University, Philadelphia, Pennsylvania, USA. -
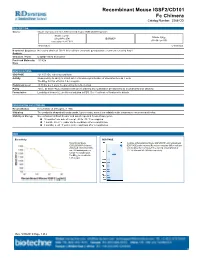
Recombinant Mouse IGSF2/CD101 Fc Chimera Catalog Number: 3368-CD
Recombinant Mouse IGSF2/CD101 Fc Chimera Catalog Number: 3368-CD DESCRIPTION Source Mouse myeloma cell line, NS0-derived mouse IGSF2/CD101 protein Mouse CD101 Mouse IgG (Gln21-Phe974) IEGRMDP 2a (Glu98-Lys330) Accession # A8E0Y8 N-terminus C-terminus N-terminal Sequence No results obtained. Gln21 inferred from enzymatic pyroglutamate treatment revealing Arg22. Analysis Structure / Form Disulfide-linked homodimer Predicted Molecular 133 kDa Mass SPECIFICATIONS SDS-PAGE 121-150 kDa, reducing conditions Activity Measured by its ability to inhibit anti-CD3-induced proliferation of stimulated human T cells. The ED50 for this effect is 1.4-8.4 μg/mL. Endotoxin Level <0.10 EU per 1 μg of the protein by the LAL method. Purity >95%, by SDS-PAGE visualized with Silver Staining and quantitative densitometry by Coomassie® Blue Staining. Formulation Lyophilized from a 0.2 μm filtered solution in PBS. See Certificate of Analysis for details. PREPARATION AND STORAGE Reconstitution Reconstitute at 400 μg/mL in PBS. Shipping The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. Stability & Storage Use a manual defrost freezer and avoid repeated freeze-thaw cycles. 12 months from date of receipt, -20 to -70 °C as supplied. 1 month, 2 to 8 °C under sterile conditions after reconstitution. 3 months, ≤ -20 °C under sterile conditions after reconstitution. DATA Bioactivity SDS-PAGE Recombinant Mouse 2 μg/lane of Recombinant Mouse IGSF2/CD101 was resolved with IGSF2/CD101 Fc Chimera SDS-PAGE under reducing (R) and non-reducing (NR) conditions (Catalog # 3368-CD) inhibits and visualized by Coomassie® Blue staining, showing bands at anti-CD3 antibody induced 121-150 kDa and 240-280 kDa, respectively. -

Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association Between BMI and Adult-Onset Non- Atopic
Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association between BMI and Adult-Onset Non- Atopic Asthma Ayoung Jeong 1,2, Medea Imboden 1,2, Akram Ghantous 3, Alexei Novoloaca 3, Anne-Elie Carsin 4,5,6, Manolis Kogevinas 4,5,6, Christian Schindler 1,2, Gianfranco Lovison 7, Zdenko Herceg 3, Cyrille Cuenin 3, Roel Vermeulen 8, Deborah Jarvis 9, André F. S. Amaral 9, Florian Kronenberg 10, Paolo Vineis 11,12 and Nicole Probst-Hensch 1,2,* 1 Swiss Tropical and Public Health Institute, 4051 Basel, Switzerland; [email protected] (A.J.); [email protected] (M.I.); [email protected] (C.S.) 2 Department of Public Health, University of Basel, 4001 Basel, Switzerland 3 International Agency for Research on Cancer, 69372 Lyon, France; [email protected] (A.G.); [email protected] (A.N.); [email protected] (Z.H.); [email protected] (C.C.) 4 ISGlobal, Barcelona Institute for Global Health, 08003 Barcelona, Spain; [email protected] (A.-E.C.); [email protected] (M.K.) 5 Universitat Pompeu Fabra (UPF), 08002 Barcelona, Spain 6 CIBER Epidemiología y Salud Pública (CIBERESP), 08005 Barcelona, Spain 7 Department of Economics, Business and Statistics, University of Palermo, 90128 Palermo, Italy; [email protected] 8 Environmental Epidemiology Division, Utrecht University, Institute for Risk Assessment Sciences, 3584CM Utrecht, Netherlands; [email protected] 9 Population Health and Occupational Disease, National Heart and Lung Institute, Imperial College, SW3 6LR London, UK; [email protected] (D.J.); [email protected] (A.F.S.A.) 10 Division of Genetic Epidemiology, Medical University of Innsbruck, 6020 Innsbruck, Austria; [email protected] 11 MRC-PHE Centre for Environment and Health, School of Public Health, Imperial College London, W2 1PG London, UK; [email protected] 12 Italian Institute for Genomic Medicine (IIGM), 10126 Turin, Italy * Correspondence: [email protected]; Tel.: +41-61-284-8378 Int. -

Datasheet: MCA2236F Product Details
Datasheet: MCA2236F Description: MOUSE ANTI HUMAN CD101:FITC Specificity: CD101 Format: FITC Product Type: Monoclonal Antibody Clone: BB27 Isotype: IgG1 Quantity: 0.1 mg Product Details Applications This product has been reported to work in the following applications. This information is derived from testing within our laboratories, peer-reviewed publications or personal communications from the originators. Please refer to references indicated for further information. For general protocol recommendations, please visit www.bio-rad-antibodies.com/protocols. Yes No Not Determined Suggested Dilution Flow Cytometry 1/5 - 1/10 Where this antibody has not been tested for use in a particular technique this does not necessarily exclude its use in such procedures. Suggested working dilutions are given as a guide only. It is recommended that the user titrates the antibody for use their own system using appropriate negative/positive controls. Target Species Human Product Form Purified IgG conjugated to Fluorescein Isothiocyanate Isomer 1 (FITC) - liquid Max Ex/Em Fluorophore Excitation Max (nm) Emission Max (nm) FITC 490 525 Preparation Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant Buffer Solution Phosphate buffered saline Preservative 0.09% Sodium Azide Stabilisers 1% Bovine Serum Albumin Approx. Protein IgG concentration 0.1 mg/ml Concentrations Immunogen Human thymic clone B12. External Database Links UniProt: Q93033 Related reagents Entrez Gene: Page 1 of 3 9398 CD101 Related reagents Synonyms EWI101, IGSF2, V7 Specificity Mouse anti Human CD101 antibody, clone BB27 recognizes human CD101, also known as Immunoglobulin superfamily member 2 (IgSF2), . Cell surface glycoprotein V7, Glu-Trp-Ile EWI motif-containing protein 101 or EWI-101. -

Human CD Marker Chart Reviewed by HLDA1 Bdbiosciences.Com/Cdmarkers
BD Biosciences Human CD Marker Chart Reviewed by HLDA1 bdbiosciences.com/cdmarkers 23-12399-01 CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD1a R4, T6, Leu6, HTA1 b-2-Microglobulin, CD74 + + + – + – – – CD93 C1QR1,C1qRP, MXRA4, C1qR(P), Dj737e23.1, GR11 – – – – – + + – – + – CD220 Insulin receptor (INSR), IR Insulin, IGF-2 + + + + + + + + + Insulin-like growth factor 1 receptor (IGF1R), IGF-1R, type I IGF receptor (IGF-IR), CD1b R1, T6m Leu6 b-2-Microglobulin + + + – + – – – CD94 KLRD1, Kp43 HLA class I, NKG2-A, p39 + – + – – – – – – CD221 Insulin-like growth factor 1 (IGF-I), IGF-II, Insulin JTK13 + + + + + + + + + CD1c M241, R7, T6, Leu6, BDCA1 b-2-Microglobulin + + + – + – – – CD178, FASLG, APO-1, FAS, TNFRSF6, CD95L, APT1LG1, APT1, FAS1, FASTM, CD95 CD178 (Fas ligand) + + + + + – – IGF-II, TGF-b latency-associated peptide (LAP), Proliferin, Prorenin, Plasminogen, ALPS1A, TNFSF6, FASL Cation-independent mannose-6-phosphate receptor (M6P-R, CIM6PR, CIMPR, CI- CD1d R3G1, R3 b-2-Microglobulin, MHC II CD222 Leukemia -

Mouse CD Marker Chart Bdbiosciences.Com/Cdmarkers
BD Mouse CD Marker Chart bdbiosciences.com/cdmarkers 23-12400-01 CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD1d CD1.1, CD1.2, Ly-38 Lipid, Glycolipid Ag + + + + + + + + CD104 Integrin b4 Laminin, Plectin + DNAX accessory molecule 1 (DNAM-1), Platelet and T cell CD226 activation antigen 1 (PTA-1), T lineage-specific activation antigen 1 CD112, CD155, LFA-1 + + + + + – + – – CD2 LFA-2, Ly-37, Ly37 CD48, CD58, CD59, CD15 + + + + + CD105 Endoglin TGF-b + + antigen (TLiSA1) Mucin 1 (MUC1, MUC-1), DF3 antigen, H23 antigen, PUM, PEM, CD227 CD54, CD169, Selectins; Grb2, β-Catenin, GSK-3β CD3g CD3g, CD3 g chain, T3g TCR complex + CD106 VCAM-1 VLA-4 + + EMA, Tumor-associated mucin, Episialin + + + + + + Melanotransferrin (MT, MTF1), p97 Melanoma antigen CD3d CD3d, CD3 d chain, T3d TCR complex + CD107a LAMP-1 Collagen, Laminin, Fibronectin + + + CD228 Iron, Plasminogen, pro-UPA (p97, MAP97), Mfi2, gp95 + + CD3e CD3e, CD3 e chain, CD3, T3e TCR complex + + CD107b LAMP-2, LGP-96, LAMP-B + + Lymphocyte antigen 9 (Ly9), -

The Evolution of the Immune System: Conservation and Diversification
Title The Evolution of the Immune System Conservation and Diversification Page left intentionally blank The Evolution of the Immune System Conservation and Diversification Davide Malagoli Department of Life Sciences Biology Building, University of Modena and Reggio Emilia, Modena, Italy AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Academic Press is an imprint of Elsevier Academic Press is an imprint of Elsevier 125 London Wall, London EC2Y 5AS, United Kingdom 525 B Street, Suite 1800, San Diego, CA 92101-4495, United States 50 Hampshire Street, 5th Floor, Cambridge, MA 02139, United States The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, UK Copyright © 2016 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek per- mission, further information about the Publisher’s permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. -

Reviewed by HLDA1
Human CD Marker Chart Reviewed by HLDA1 T Cell Key Markers CD3 CD4 CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD8 CD1a R4, T6, Leu6, HTA1 b-2-Microglobulin, CD74 + + + – + – – – CD74 DHLAG, HLADG, Ia-g, li, invariant chain HLA-DR, CD44 + + + + + + CD158g KIR2DS5 + + CD248 TEM1, Endosialin, CD164L1, MGC119478, MGC119479 Collagen I/IV Fibronectin + ST6GAL1, MGC48859, SIAT1, ST6GALL, ST6N, ST6 b-Galactosamide a-2,6-sialyl- CD1b R1, T6m Leu6 b-2-Microglobulin + + + – + – – – CD75 CD22 CD158h KIR2DS1, p50.1 HLA-C + + CD249 APA, gp160, EAP, ENPEP + + tranferase, Sialo-masked lactosamine, Carbohydrate of a2,6 sialyltransferase + – – + – – + – – CD1c M241, R7, T6, Leu6, BDCA1 b-2-Microglobulin + + + – + – – – CD75S a2,6 Sialylated lactosamine CD22 (proposed) + + – – + + – + + + CD158i KIR2DS4, p50.3 HLA-C + – + CD252 TNFSF4, -

CD System of Surface Molecules
THE CD SYSTEM OF LEUKOCYTE APPENDIX 4A SURFACE MOLECULES Monoclonal Antibodies to Human Cell Surface Antigens APPENDIX 4A Alice Beare,1 Hannes Stockinger,2 Heddy Zola,1 and Ian Nicholson1 1Women’s and Children’s Health Research Institute, Women’s and Children’s Hospital, Adelaide, Australia 2Institute of Immunology, University of Vienna, Vienna ABSTRACT Many of the leukocyte cell surface molecules are known by “CD” numbers. In this Appendix, a short introduction describes the history and the use of CD nomenclature and provides a few key references to enable access to the wider literature. This is followed by a table that lists all human molecules with approved CD names, tabulating alternative names, key structural features, cellular expression, major known functions, and usefulness of the molecules or antibodies against them in research or clinical applications. Curr. Protoc. Immunol. 80:A.4A.1-A.4A.73. C 2008 by John Wiley & Sons, Inc. Keywords: CD nomenclature r HLDA r HCDM r leukocyte marker r human leukocyte differentiation r antigens INTRODUCTION During the last 25 years, large numbers of monoclonal antibodies (MAbs) have been pro- duced that have facilitated the purification and functional characterization of a plethora of leukocyte surface molecules. The antibodies have been even more useful as markers for cell populations, allowing the counting, separation, and functional study of numer- ous subsets of cells of the immune system. A series of international workshops were instrumental in coordinating this development through multi-laboratory “blind” studies of thousands of antibodies. These HLDA (Human Leukocyte Differentiation Antigens) Workshops have, up until now, defined 500 different entities and assigned them cluster of differentiation (CD) designations. -

Contact Biolegend
Human CD Molecules CD Antigen OtherNames MolecularWeight (kD) CellularExpression Ligand/Receptor/Association Functions IntracellularInteraction CD Antigen OtherNames MolecularWeight (kD) CellularExpression Ligand/Receptor/Association Functions IntracellularInteraction CD Antigen OtherNames MolecularWeight (kD) CellularExpression Ligand/Receptor/Association Functions IntracellularInteraction CD Antigen OtherNames MolecularWeight (kD) CellularExpression Ligand/Receptor/Association Functions IntracellularInteraction CD1a R4, T6, HTA1 49 cortical thy-c, CD1-restricted TCR lipid Ag presentation ß2m, CD8 CD72 Lyb-2 39 B (except plasma), CD5, CD100 B activ and prolif Grb2, SHP1, BLNK CD158e2 KIR3DS1, NKAT10 70 NK, some T HLA-Bw4 activates NK cytotoxicity CDK3 CD268 BAFFR, BR3, 19 B, T sub BAFF B survival and TRAF3, PMPCB, NFκB Langerhans, DC mac, FDC, T sub TNFRSF13c maturation, T activ CD1b R1, T6 45 cortical thy-c, CD1-restricted TCR lipid Ag presentation ß2m, CD8 CD73 Ecto-5'- 70 T sub, B sub, FDC, epi, endo AMP dephosphorylation, β-Actin, Fibronectin CD158f KIR2DL5A NK, some T HLA-B inhibits NK cytotoxicity SHP1, SHP2 CD269 BCMA, TNFRSF17 B, plasma cells BAFF, APRIL plasma cell survival TRAF1-3 Langerhans, DC nucleotidase costim, adh 1, Laminin A CD1c BDCA-1, R7, T6 43 cortical thy-c, CD1-restricted TCR lipid Ag presentation ß2m, CD8 CD74 Ii, invariant chain 33,35,41 B, act T, mac, Langerhans, CD44, MHC class II, MIF intracellular sorting of Cathepsin L, CD1d CD158g KIR2DS5 NK, some T activates NK cytotoxicity CD270 HVEM, HVEA, TR2, T, B, -

Ep 2863222 A1
(19) TZZ ¥ _T (11) EP 2 863 222 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 22.04.2015 Bulletin 2015/17 G01N 33/53 (2006.01) G01N 33/567 (2006.01) (21) Application number: 14189047.5 (22) Date of filing: 06.03.2007 (84) Designated Contracting States: • Scholle, Michael, D AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Bolingbrook, IL 60440 (US) HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE • Popkov, Mikhail SI SK TR San Diego, CA 92131 (US) • Gordon, Nathaniel, C. (30) Priority: 06.03.2006 US 743410 P Cambridge, CB4 1AU (GB) 21.03.2006 US 743622 P • Crameri, Andreas 27.09.2006 US 528927 Hitchin 27.09.2006 US 528950 Central Bedfordshire, SG5 3LE (GB) (62) Document number(s) of the earlier application(s) in (74) Representative: Kremer, Simon Mark et al accordance with Art. 76 EPC: Mewburn Ellis LLP 11172812.7 / 2 402 754 33 Gutter Lane 07752636.6 / 1 996 220 London EC2V 8AS (GB) (71) Applicant: Amunix Operating Inc. Mountain View, CA 94043 (US) Remarks: •This application was filed on 15-10-2014 as a (72) Inventors: divisional application to the application mentioned • Schellenberger, Volker under INID code 62. Palo Alto, CA 94303 (US) •Claims filed after the date of filing of the application/ • Stemmer, Willem, P after the date of receipt of the divisional application Los Gatos, CA 95030 (US) (Rule 68(4) EPC). •Wang,Chia- wei Santa Clara, CA 95051 (US) (54) Unstructured recombinant polymers and uses thereof (57) The present invention provides unstructured re- binant polypeptides including vectors encoding the sub- combinant polymers (URPs) and proteins containing one ject proteinaceous entities, as well as host cells compris- or more of the URPs.