WO 2013/184938 A2 12 December 2013 (12.12.2013) P O P C T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

Chronic Myeloid Leukemia: Mechanisms of Blastic Transformation
Chronic myeloid leukemia: mechanisms of blastic transformation Danilo Perrotti, … , John Goldman, Tomasz Skorski J Clin Invest. 2010;120(7):2254-2264. https://doi.org/10.1172/JCI41246. Science in Medicine The BCR-ABL1 oncoprotein transforms pluripotent HSCs and initiates chronic myeloid leukemia (CML). Patients with early phase (also known as chronic phase [CP]) disease usually respond to treatment with ABL tyrosine kinase inhibitors (TKIs), although some patients who respond initially later become resistant. In most patients, TKIs reduce the leukemia cell load substantially, but the cells from which the leukemia cells are derived during CP (so-called leukemia stem cells [LSCs]) are intrinsically insensitive to TKIs and survive long term. LSCs or their progeny can acquire additional genetic and/or epigenetic changes that cause the leukemia to transform from CP to a more advanced phase, which has been subclassified as either accelerated phase or blastic phase disease. The latter responds poorly to treatment and is usually fatal. Here, we discuss what is known about the molecular mechanisms leading to blastic transformation of CML and propose some novel therapeutic approaches. Find the latest version: https://jci.me/41246/pdf Science in medicine Chronic myeloid leukemia: mechanisms of blastic transformation Danilo Perrotti,1 Catriona Jamieson,2 John Goldman,3 and Tomasz Skorski4 1Department of Molecular Virology, Immunology and Medical Genetics and Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio, USA. 2Division of Hematology-Oncology, Department of Internal Medicine, University of California at San Diego, La Jolla, California, USA. 3Department of Haematology, Imperial College at Hammersmith Hospital, London, United Kingdom. 4Department of Microbiology and Immunology, Temple University, Philadelphia, Pennsylvania, USA. -

Differential Proteomic Analysis of the Pancreas of Diabetic Db/Db Mice Reveals the Proteins Involved in the Development of Complications of Diabetes Mellitus
Int. J. Mol. Sci. 2014, 15, 9579-9593; doi:10.3390/ijms15069579 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Article Differential Proteomic Analysis of the Pancreas of Diabetic db/db Mice Reveals the Proteins Involved in the Development of Complications of Diabetes Mellitus Victoriano Pérez-Vázquez 1,*, Juan M. Guzmán-Flores 1, Daniela Mares-Álvarez 1, Magdalena Hernández-Ortiz 2, Maciste H. Macías-Cervantes 1, Joel Ramírez-Emiliano 1 and Sergio Encarnación-Guevara 2 1 Depto. de Ciencias Médicas, División de Ciencias de la Salud, Campus León, Universidad de Guanajuato, León, Guanajuato 37320, Mexico; E-Mails: [email protected] (J.M.G.-F.); [email protected] (D.M.-A.); [email protected] (M.H.M.-C.); [email protected] (J.R.-E.) 2 Centro de Ciencias Genómicas, Universidad Nacional Autónoma de México, Cuernavaca, Morelos 62210, Mexico; E-Mails: [email protected] (M.H.-O.); [email protected] (S.E.-G.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +52-477-7143-812; Fax: +52-477-7167-623. Received: 4 April 2014; in revised form: 14 May 2014 / Accepted: 19 May 2014 / Published: 30 May 2014 Abstract: Type 2 diabetes mellitus is characterized by hyperglycemia and insulin-resistance. Diabetes results from pancreatic inability to secrete the insulin needed to overcome this resistance. We analyzed the protein profile from the pancreas of ten-week old diabetic db/db and wild type mice through proteomics. Pancreatic proteins were separated in two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) and significant changes in db/db mice respect to wild type mice were observed in 27 proteins. -

ONLINE SUPPLEMENTARY TABLE Table 2. Differentially Expressed
ONLINE SUPPLEMENTARY TABLE Table 2. Differentially Expressed Probe Sets in Livers of GK Rats. A. Immune/Inflammatory (67 probe sets, 63 genes) Age Strain Probe ID Gene Name Symbol Accession Gene Function 5 WKY 1398390_at small inducible cytokine B13 precursor Cxcl13 AA892854 chemokine activity; lymph node development 5 WKY 1389581_at interleukin 33 Il33 BF390510 cytokine activity 5 WKY *1373970_at interleukin 33 Il33 AI716248 cytokine activity 5 WKY 1369171_at macrophage stimulating 1 (hepatocyte growth factor-like) Mst1; E2F2 NM_024352 serine-throenine kinase; tumor suppression 5 WKY 1388071_x_at major histocompatability antigen Mhc M24024 antigen processing and presentation 5 WKY 1385465_at sialic acid binding Ig-like lectin 5 Siglec5 BG379188 sialic acid-recognizing receptor 5 WKY 1393108_at major histocompatability antigen Mhc BM387813 antigen processing and presentation 5 WKY 1388202_at major histocompatability antigen Mhc BI395698 antigen processing and presentation 5 WKY 1371171_at major histocompatability antigen Mhc M10094 antigen processing and presentation 5 WKY 1370382_at major histocompatability antigen Mhc BI279526 antigen processing and presentation 5 WKY 1371033_at major histocompatability antigen Mhc AI715202 antigen processing and presentation 5 WKY 1383991_at leucine rich repeat containing 8 family, member E Lrrc8e BE096426 proliferation and activation of lymphocytes and monocytes. 5 WKY 1383046_at complement component factor H Cfh; Fh AA957258 regulation of complement cascade 4 WKY 1369522_a_at CD244 natural killer -

Serine Proteases with Altered Sensitivity to Activity-Modulating
(19) & (11) EP 2 045 321 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.04.2009 Bulletin 2009/15 C12N 9/00 (2006.01) C12N 15/00 (2006.01) C12Q 1/37 (2006.01) (21) Application number: 09150549.5 (22) Date of filing: 26.05.2006 (84) Designated Contracting States: • Haupts, Ulrich AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 51519 Odenthal (DE) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • Coco, Wayne SK TR 50737 Köln (DE) •Tebbe, Jan (30) Priority: 27.05.2005 EP 05104543 50733 Köln (DE) • Votsmeier, Christian (62) Document number(s) of the earlier application(s) in 50259 Pulheim (DE) accordance with Art. 76 EPC: • Scheidig, Andreas 06763303.2 / 1 883 696 50823 Köln (DE) (71) Applicant: Direvo Biotech AG (74) Representative: von Kreisler Selting Werner 50829 Köln (DE) Patentanwälte P.O. Box 10 22 41 (72) Inventors: 50462 Köln (DE) • Koltermann, André 82057 Icking (DE) Remarks: • Kettling, Ulrich This application was filed on 14-01-2009 as a 81477 München (DE) divisional application to the application mentioned under INID code 62. (54) Serine proteases with altered sensitivity to activity-modulating substances (57) The present invention provides variants of ser- screening of the library in the presence of one or several ine proteases of the S1 class with altered sensitivity to activity-modulating substances, selection of variants with one or more activity-modulating substances. A method altered sensitivity to one or several activity-modulating for the generation of such proteases is disclosed, com- substances and isolation of those polynucleotide se- prising the provision of a protease library encoding poly- quences that encode for the selected variants. -
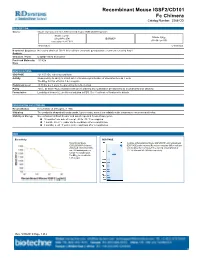
Recombinant Mouse IGSF2/CD101 Fc Chimera Catalog Number: 3368-CD
Recombinant Mouse IGSF2/CD101 Fc Chimera Catalog Number: 3368-CD DESCRIPTION Source Mouse myeloma cell line, NS0-derived mouse IGSF2/CD101 protein Mouse CD101 Mouse IgG (Gln21-Phe974) IEGRMDP 2a (Glu98-Lys330) Accession # A8E0Y8 N-terminus C-terminus N-terminal Sequence No results obtained. Gln21 inferred from enzymatic pyroglutamate treatment revealing Arg22. Analysis Structure / Form Disulfide-linked homodimer Predicted Molecular 133 kDa Mass SPECIFICATIONS SDS-PAGE 121-150 kDa, reducing conditions Activity Measured by its ability to inhibit anti-CD3-induced proliferation of stimulated human T cells. The ED50 for this effect is 1.4-8.4 μg/mL. Endotoxin Level <0.10 EU per 1 μg of the protein by the LAL method. Purity >95%, by SDS-PAGE visualized with Silver Staining and quantitative densitometry by Coomassie® Blue Staining. Formulation Lyophilized from a 0.2 μm filtered solution in PBS. See Certificate of Analysis for details. PREPARATION AND STORAGE Reconstitution Reconstitute at 400 μg/mL in PBS. Shipping The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. Stability & Storage Use a manual defrost freezer and avoid repeated freeze-thaw cycles. 12 months from date of receipt, -20 to -70 °C as supplied. 1 month, 2 to 8 °C under sterile conditions after reconstitution. 3 months, ≤ -20 °C under sterile conditions after reconstitution. DATA Bioactivity SDS-PAGE Recombinant Mouse 2 μg/lane of Recombinant Mouse IGSF2/CD101 was resolved with IGSF2/CD101 Fc Chimera SDS-PAGE under reducing (R) and non-reducing (NR) conditions (Catalog # 3368-CD) inhibits and visualized by Coomassie® Blue staining, showing bands at anti-CD3 antibody induced 121-150 kDa and 240-280 kDa, respectively. -

Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association Between BMI and Adult-Onset Non- Atopic
Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association between BMI and Adult-Onset Non- Atopic Asthma Ayoung Jeong 1,2, Medea Imboden 1,2, Akram Ghantous 3, Alexei Novoloaca 3, Anne-Elie Carsin 4,5,6, Manolis Kogevinas 4,5,6, Christian Schindler 1,2, Gianfranco Lovison 7, Zdenko Herceg 3, Cyrille Cuenin 3, Roel Vermeulen 8, Deborah Jarvis 9, André F. S. Amaral 9, Florian Kronenberg 10, Paolo Vineis 11,12 and Nicole Probst-Hensch 1,2,* 1 Swiss Tropical and Public Health Institute, 4051 Basel, Switzerland; [email protected] (A.J.); [email protected] (M.I.); [email protected] (C.S.) 2 Department of Public Health, University of Basel, 4001 Basel, Switzerland 3 International Agency for Research on Cancer, 69372 Lyon, France; [email protected] (A.G.); [email protected] (A.N.); [email protected] (Z.H.); [email protected] (C.C.) 4 ISGlobal, Barcelona Institute for Global Health, 08003 Barcelona, Spain; [email protected] (A.-E.C.); [email protected] (M.K.) 5 Universitat Pompeu Fabra (UPF), 08002 Barcelona, Spain 6 CIBER Epidemiología y Salud Pública (CIBERESP), 08005 Barcelona, Spain 7 Department of Economics, Business and Statistics, University of Palermo, 90128 Palermo, Italy; [email protected] 8 Environmental Epidemiology Division, Utrecht University, Institute for Risk Assessment Sciences, 3584CM Utrecht, Netherlands; [email protected] 9 Population Health and Occupational Disease, National Heart and Lung Institute, Imperial College, SW3 6LR London, UK; [email protected] (D.J.); [email protected] (A.F.S.A.) 10 Division of Genetic Epidemiology, Medical University of Innsbruck, 6020 Innsbruck, Austria; [email protected] 11 MRC-PHE Centre for Environment and Health, School of Public Health, Imperial College London, W2 1PG London, UK; [email protected] 12 Italian Institute for Genomic Medicine (IIGM), 10126 Turin, Italy * Correspondence: [email protected]; Tel.: +41-61-284-8378 Int. -

Datasheet: MCA2236F Product Details
Datasheet: MCA2236F Description: MOUSE ANTI HUMAN CD101:FITC Specificity: CD101 Format: FITC Product Type: Monoclonal Antibody Clone: BB27 Isotype: IgG1 Quantity: 0.1 mg Product Details Applications This product has been reported to work in the following applications. This information is derived from testing within our laboratories, peer-reviewed publications or personal communications from the originators. Please refer to references indicated for further information. For general protocol recommendations, please visit www.bio-rad-antibodies.com/protocols. Yes No Not Determined Suggested Dilution Flow Cytometry 1/5 - 1/10 Where this antibody has not been tested for use in a particular technique this does not necessarily exclude its use in such procedures. Suggested working dilutions are given as a guide only. It is recommended that the user titrates the antibody for use their own system using appropriate negative/positive controls. Target Species Human Product Form Purified IgG conjugated to Fluorescein Isothiocyanate Isomer 1 (FITC) - liquid Max Ex/Em Fluorophore Excitation Max (nm) Emission Max (nm) FITC 490 525 Preparation Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant Buffer Solution Phosphate buffered saline Preservative 0.09% Sodium Azide Stabilisers 1% Bovine Serum Albumin Approx. Protein IgG concentration 0.1 mg/ml Concentrations Immunogen Human thymic clone B12. External Database Links UniProt: Q93033 Related reagents Entrez Gene: Page 1 of 3 9398 CD101 Related reagents Synonyms EWI101, IGSF2, V7 Specificity Mouse anti Human CD101 antibody, clone BB27 recognizes human CD101, also known as Immunoglobulin superfamily member 2 (IgSF2), . Cell surface glycoprotein V7, Glu-Trp-Ile EWI motif-containing protein 101 or EWI-101. -

Handbook of Proteolytic Enzymes Second Edition Volume 1 Aspartic and Metallo Peptidases
Handbook of Proteolytic Enzymes Second Edition Volume 1 Aspartic and Metallo Peptidases Alan J. Barrett Neil D. Rawlings J. Fred Woessner Editor biographies xxi Contributors xxiii Preface xxxi Introduction ' Abbreviations xxxvii ASPARTIC PEPTIDASES Introduction 1 Aspartic peptidases and their clans 3 2 Catalytic pathway of aspartic peptidases 12 Clan AA Family Al 3 Pepsin A 19 4 Pepsin B 28 5 Chymosin 29 6 Cathepsin E 33 7 Gastricsin 38 8 Cathepsin D 43 9 Napsin A 52 10 Renin 54 11 Mouse submandibular renin 62 12 Memapsin 1 64 13 Memapsin 2 66 14 Plasmepsins 70 15 Plasmepsin II 73 16 Tick heme-binding aspartic proteinase 76 17 Phytepsin 77 18 Nepenthesin 85 19 Saccharopepsin 87 20 Neurosporapepsin 90 21 Acrocylindropepsin 9 1 22 Aspergillopepsin I 92 23 Penicillopepsin 99 24 Endothiapepsin 104 25 Rhizopuspepsin 108 26 Mucorpepsin 11 1 27 Polyporopepsin 113 28 Candidapepsin 115 29 Candiparapsin 120 30 Canditropsin 123 31 Syncephapepsin 125 32 Barrierpepsin 126 33 Yapsin 1 128 34 Yapsin 2 132 35 Yapsin A 133 36 Pregnancy-associated glycoproteins 135 37 Pepsin F 137 38 Rhodotorulapepsin 139 39 Cladosporopepsin 140 40 Pycnoporopepsin 141 Family A2 and others 41 Human immunodeficiency virus 1 retropepsin 144 42 Human immunodeficiency virus 2 retropepsin 154 43 Simian immunodeficiency virus retropepsin 158 44 Equine infectious anemia virus retropepsin 160 45 Rous sarcoma virus retropepsin and avian myeloblastosis virus retropepsin 163 46 Human T-cell leukemia virus type I (HTLV-I) retropepsin 166 47 Bovine leukemia virus retropepsin 169 48 -

Human CD Marker Chart Reviewed by HLDA1 Bdbiosciences.Com/Cdmarkers
BD Biosciences Human CD Marker Chart Reviewed by HLDA1 bdbiosciences.com/cdmarkers 23-12399-01 CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD1a R4, T6, Leu6, HTA1 b-2-Microglobulin, CD74 + + + – + – – – CD93 C1QR1,C1qRP, MXRA4, C1qR(P), Dj737e23.1, GR11 – – – – – + + – – + – CD220 Insulin receptor (INSR), IR Insulin, IGF-2 + + + + + + + + + Insulin-like growth factor 1 receptor (IGF1R), IGF-1R, type I IGF receptor (IGF-IR), CD1b R1, T6m Leu6 b-2-Microglobulin + + + – + – – – CD94 KLRD1, Kp43 HLA class I, NKG2-A, p39 + – + – – – – – – CD221 Insulin-like growth factor 1 (IGF-I), IGF-II, Insulin JTK13 + + + + + + + + + CD1c M241, R7, T6, Leu6, BDCA1 b-2-Microglobulin + + + – + – – – CD178, FASLG, APO-1, FAS, TNFRSF6, CD95L, APT1LG1, APT1, FAS1, FASTM, CD95 CD178 (Fas ligand) + + + + + – – IGF-II, TGF-b latency-associated peptide (LAP), Proliferin, Prorenin, Plasminogen, ALPS1A, TNFSF6, FASL Cation-independent mannose-6-phosphate receptor (M6P-R, CIM6PR, CIMPR, CI- CD1d R3G1, R3 b-2-Microglobulin, MHC II CD222 Leukemia -

Time-Course Microarray Analysis for Identifying Candidate Genes
Bae et al. Genes & Nutrition (2016) 11:30 DOI 10.1186/s12263-016-0547-x RESEARCH Open Access Time-course microarray analysis for identifying candidate genes involved in obesity-associated pathological changes in the mouse colon Yun Jung Bae1†, Sung-Eun Kim2†, Seong Yeon Hong2, Taesun Park3,6, Sang Gyu Lee4, Myung-Sook Choi5,6 and Mi-Kyung Sung2,6* Abstract Background: Obesity is known to increase the risk of colorectal cancer. However, mechanisms underlying the pathogenesis of obesity-induced colorectal cancer are not completely understood. The purposes of this study were to identify differentially expressed genes in the colon of mice with diet-induced obesity and to select candidate genes as early markers of obesity-associated abnormal cell growth in the colon. Methods: C57BL/6N mice were fed normal diet (11% fat energy) or high-fat diet (40% fat energy) and were euthanized at different time points. Genome-wide expression profiles of the colon were determined at 2, 4, 8, and 12 weeks. Cluster analysis was performed using expression data of genes showing log2 fold change of ≥1 or ≤−1 (twofold change), based on time-dependent expression patterns, followed by virtual network analysis. Results: High-fat diet-fed mice showed significant increase in body weight and total visceral fat weight over 12 weeks. Time-course microarray analysis showed that 50, 47, 36, and 411 genes were differentially expressed at 2, 4, 8, and 12 weeks, respectively. Ten cluster profiles representing distinguishable patterns of genes differentially expressed over time were determined. Cluster 4, which consisted of genes showing the most significant alterations in expression in response to high-fat diet over 12 weeks, included Apoa4 (apolipoprotein A-IV), Ppap2b (phosphatidic acid phosphatase type 2B), Cel (carboxyl ester lipase), and Clps (colipase, pancreatic), which interacted strongly with surrounding genes associated with colorectal cancer or obesity. -

( 12 ) United States Patent
US010245229B2 (12 ) United States Patent ( 10 ) Patent No. : US 10 , 245 ,229 B2 Heartlein et al. (45 ) Date of Patent: Apr. 2 , 2019 ( 54 ) PULMONARY DELIVERY OF MRNA TO ( 56 ) References Cited NON -LUNG TARGET CELLS U . S . PATENT DOCUMENTS ( 71 ) Applicants : Shire Human Genetic Therapies, Inc. , 5 ,705 , 385 A 1 / 1998 Bally et al . Lexington , MA (US ) ; Ethris GmbH , 5 , 965, 434 A 10 / 1999 Wolff et al . Martinsried (DE ) 5 , 976 , 567 A 11 / 1999 Wheeler et al. 5 , 981 ,501 A 11/ 1999 Wheeler et al. 6 ,077 ,835 A 6 / 2000 Hanson et al . (72 ) Inventors : Michael Heartlein , Lexington , MA 6 ,534 , 484 B1 3 / 2003 Wheeler et al . (US ) ; Braydon Charles Guild , 6 ,734 , 171 B1 5 /2004 Saravolac et al. Lexington , MA (US ) ; Frank DeRosa , 6 , 815 ,432 B2 11/ 2004 Wheeler et al . Lexington , MA (US ) ; Carsten 7 , 422 , 902 B1 9 / 2008 Wheeler et al . Rudolph , Krailling ( DE ) ; Christian 7 ,745 ,651 B2 6 /2010 Heyes et al. 7 ,799 , 565 B2 9 /2010 MacLachlan et al. Plank , Wessling (DE ) 7 , 803 ,397 B2 9 / 2010 Heyes et al . 7 , 901, 708 B2 3 / 2011 MacLachlan et al. (73 ) Assignees : Translate Bio , Inc . , Lexington , MA 8 , 101 ,741 B2 1 / 2012 MacLachlan et al . (US ) ; Ethris GmbH , Martinsried (DE ) 8 , 188 , 263 B2 5 / 2012 MacLachlan et al . 8 , 236 , 943 B2 8 / 2012 Lee et al. 8 ,329 ,070 B2 12 /2012 MacLachlan et al . ( * ) Notice : Subject to any disclaimer , the term of this 8 ,513 ,403 B2 8 / 2013 MacLachlan et al.