Ems Medication Formularies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thiosulfate De Sodium Sterop
Thiosulfate de Sodium Sterop 1. DENOMINATION DU MEDICAMENT THIOSULFATE DE SODIUM STEROP 1g/5ml solution injectable 2. COMPOSITION QUALITATIVE ET QUANTITATIVE Substance active : Une ampoule de 5ml contient 1g de thiosulfate de sodium dans de l’eau pour préparations injectables. 1 ml de solution contient 200mg de thiosulfate de sodium. Pour la liste complète des excipients, voir rubrique 6.1. 3. FORME PHARMACEUTIQUE Solution injectable. 4. DONNEES CLINIQUES 4.1 Indications thérapeutiques - Antidote dans le traitement des intoxications par les cyanures ainsi que par le nitroprussiate de sodium. - Prévention des effets néphrotoxiques du cisplatine. Thiosulfate de Sodium Sterop © Pharma.be Pagina 1 van 6 4.2 Posologie et mode d’administration Mode d'administration Pour voie intraveineuse lente. Posologie Intoxications par les cyanures: Adultes: La posologie habituelle est de 12,5 g de thiosulfate de sodium, administré par voie I.V. sur une période de 5 à 10 minutes. Enfants: La posologie habituelle est de 412,5 mg de thiosulfate de sodium par kg, administré par voie I.V. à la vitesse de 0,625 à 1,25 g/minute sur une période de 5 à 10 minutes. Si les symptômes persistent ou réapparaissent dans la demi-heure ou dans l’heure ayant suivi la première injection, une seconde injection de thiosulfate de sodium est nécessaire avec comme posologie la moitié de celle de la première injection. En prévention des effets néphrotoxiques du cisplatine: Chez l'adulte, la posologie initiale est de 9 g de thiosulfate de sodium par m2 de surface corporelle, administré par voie I.V. Elle est suivie par l'injection de 1,2 g de thiosulfate de sodium par m2 de surface corporelle par heure, en perfusion I.V. -

Adult BLS Standing Orders • Ensure EMS Provider Safety, Consider HAZMAT Activation
Imperial County Public Health Department Emergency Medical Services Agency Policy/Procedure/Protocol Manual Treatment Protocols Date: 07/01/2021 Poisoning/Intoxication/Envenomation - Adult Policy #9160A Adult BLS Standing Orders • Ensure EMS provider safety, consider HAZMAT activation. Recognize, Notify, Isolate • Universal Patient Protocol • Do not approach patient or location if scene safety is in question • Obtain accurate history of incident: o Name of product or substance o Quantity ingested, and/or duration of exposure o Time elapsed since exposure o If safe and accessible, bring medications or bottles to hospital • Move victim(s) to safe environment • Externally decontaminate - PRN • Continuously monitor ECG, blood pressure, pulse oximetry, and capnography (if ALS present) PRN • Give oxygen and provide airway support per Airway Policy • Contact Poison Control Center as needed 1 (800) 222-1222 Suspected Opioid Overdose with Respirations <12 RPM • If possible, avoid the use of a supraglottic device prior to the administration of naloxone • Administer naloxone 0.1 mg/kg, max of 2 mg IN. May repeat up to three (3) times, q5min • May assist family/friends on-scene with administration of patient’s own naloxone • NOTE - Use with caution in opioid dependent pain management patients • Assess vitals, with specific attention to respiratory rate and respiratory drive • Note pupil exam • Note drug paraphernalia or medication bottles near patient Suspected Stimulant Overdose with Sudden Hypoventilation, Oxygen Desaturation, or Apnea • High flow -

Hydrogen Sulfide Metabolite, Sodium Thiosulfate
International Journal of Molecular Sciences Review Hydrogen Sulfide Metabolite, Sodium Thiosulfate: Clinical Applications and Underlying Molecular Mechanisms Max Y. Zhang 1,2, George J. Dugbartey 1,2,3, Smriti Juriasingani 1,3 and Alp Sener 1,2,3,4,* 1 Matthew Mailing Center for Translational Transplant Studies, London Health Sciences Center, Western University, London, ON N6A 5A5, Canada; [email protected] (M.Y.Z.); [email protected] (G.J.D.); [email protected] (S.J.) 2 London Health Sciences Center, Multi-Organ Transplant Program, Western University, London, ON N6A 5A5, Canada 3 London Health Sciences Center, Department of Surgery, Division of Urology, Western University, London, ON N6A 5A5, Canada 4 Department of Microbiology & Immunology, Schulich School of Medicine & Dentistry, University of Western Ontario, London, ON N6A 3K7, Canada * Correspondence: [email protected]; Tel.: +1(519) 6633352 Abstract: Thiosulfate in the form of sodium thiosulfate (STS) is a major oxidation product of hydrogen sulfide (H2S), an endogenous signaling molecule and the third member of the gasotransmitter family. STS is currently used in the clinical treatment of acute cyanide poisoning, cisplatin toxicities in cancer therapy, and calciphylaxis in dialysis patients. Burgeoning evidence show that STS has antioxidant and anti-inflammatory properties, making it a potential therapeutic candidate molecule that can target multiple molecular pathways in various diseases and drug-induced toxicities. This review Citation: Zhang, M.Y.; Dugbartey, discusses the biochemical and molecular pathways in the generation of STS from H2S, its clinical G.J.; Juriasingani, S.; Sener, A. usefulness, and potential clinical applications, as well as the molecular mechanisms underlying these Hydrogen Sulfide Metabolite, clinical applications and a future perspective in kidney transplantation. -

Ingestions, Intoxications, and the Critically Ill Child Poisoning in Children
Ingestions, Intoxications, and the Critically Ill Child Poisoning in Children • 1 million cases of exposure to toxins in children younger than 6 years reported in the U.S. In 1993 • estimated that another 1 million exposures to toxins not reported • 1% have moderate or major life-threatening symptoms • 60-100 deaths annually in the U.S Poisoning in Children Less Than 5 Years Old • accounts for 85-90% of pediatric poisoning • is generally accidental • secondary to exploratory behavior and lack of supervision • tend to involve single agent ingestions Poisoning in Children Over 5 Years Old • accounts for 10-15% of pediatric poisoning • is generally intentional • secondary to suicide attempts or gestures, or to intoxications and inadvertent overdose • tend to involve multiple agent ingestions General Concepts for Pediatric Poisoning • Prevention • Initial Stabilization • Diagnosis • Specific Antidotes Management of the Poisoned Child • Treat the Patient, Not the Poison --patient-specific treatment is safer, less expensive, and more effective Management of the Poisoned Child • Stabilization --Airway --Breathing --Circulation --Disability (neurologic) Management of the Poisoned Child • Respiratory Failure --airway obstruction from secretions, refluxed gastric contents, airway muscle relaxation --respiratory muscle rigidity --loss of respiratory drive --pulmonary edema Management of the Poisoned Child • Cardiovascular Collapse --arteriolar dilation --venous dilation --myocardial depression --dysrhythmias Management of the Poisoned Child • Neurologic -

Antidote List
Antidote List Recommended minimum amounts for hospital pharmacies to stock Call the Maryland Poison Center for expert advice on the treatment of all poisonings and overdoses: 1-800-222-1222 Poisoning Minimum Stocking Antidote Indication Recommendations Oral: 120 grams Acetylcysteine Acetaminophen IV: 96 grams Crotaline snake Antivenin, snake (CroFab®) 12 vials envenomation 1 vial Black widow spider (Note: Merck limits distribution Antivenin, black widow spider envenomation to cases of confirmed bites with symptoms only) Organophosphate & Atropine carbamate insecticides, nerve 165 mg gases Calcium chloride Calcium channel blockers 10 grams 2.25 grams Calcium disodium EDTA Lead (Available direct from ASD Specialty Healthcare) Hydrofluoric acid 1 kg (powder) Calcium gluconate Calcium channel blockers IV: 30 grams 1 kit Cyanide Antidote Kit Cyanide (or Hydroxocobalamin, see below) Cyproheptadine (Periactin®) Serotonin syndrome 80 mg Neuroleptic malignant Dantrolene syndrome, stimulant-induced 720 mg hyperthermia Deferoxamine mesylate Iron 8 grams (Desferal®) Digoxin Immune FAB Digoxin 20 vials (Digibind®, DigiFab®) Dimercaprol (BAL) Heavy metals 1.5 grams 1 | P a g e Maryland Poison Center Antidote List – continued Poisoning Minimum Stocking Antidote Indication Recommendations DMSA (Succimer, Chemet®) Heavy metals 2000 mg Folic acid Methanol IV: 150 mg Flumazenil (Romazicon®) Benzodiazepines 10 mg Fomepizole (Antizol®) Ethylene glycol, methanol 12 grams Beta blockers, Glucagon 50 mg calcium channel blockers Hydroxocobalamin (Cyanokit®) Cyanide -
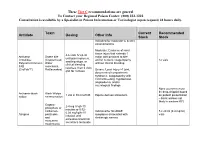
Antidote Stocking Tier C
These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Toxin Current Recommended Antidote Dosing Other Info Stock Stock Indicated for moderate to severe envenomations Moderate: Evidence of local tissue injury that extends 1 4-6 vials IV q2-4h Antivenin Snake bite major joint proximal to bite until pain improves, Crotalidae (Copperhead, and/or numeric coagulopathy 12 vials swelling stops, or Polyvalent Immune Water without clinical bleeding clinical bleeding FAB moccasins, resolves, then 2 vials (CroFab™) Rattlesnakes) Severe: Local injury >1 joint, q6h for 3 doses documented compartment syndrome, coagulopathy with clinical bleeding, hypotension, angioedema, and/or neurological findings None (currently must be drop-shipped based Antivenin-black Black Widow 1 vial in 50 ml of NS Equine derived antivenom on patient presentation widow envenomation – black widows not likely in western KY) Organo- 2-4 mg IV q5-10 phosphate or minutes or 0.02- carbamate Indicated for SLUDGE 5 x 20 ml (0.4 mg/ml) 0.08 mg/kg/hr IV Atropine pesticides symptoms associated with vials infusion until and cholinergic excess excessive bronchial muscarine secretions terminate mushrooms These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Antidote Current Recommended Toxin -

Dialyzability of Medications During Intermittent Hemodialysis
DialyzeIHD: Dialyzability of Medications During Intermittent Hemodialysis % Dialyzed % Dialyzed % Dialyzed % Dialyzed IHD Dosing; Administration IHD Dosing; Administration IHD Dosing; Administration IHD Dosing; Administration Timing Drug (Type of Drug (Type of Drug (Type of Drug (Type of Timing Around HD Session Timing Around HD Session Around HD Session Dialyzer) Timing Around HD Session Dialyzer) Dialyzer) Dialyzer) 0.25-0.5mg PO Q8H PRN, Insulin Aspart, Pentamidine 0 4mg/kg IV Q24-36H, Not recommended for use, Clonazepam N/A Reduce to 25-50% of normal dose Acarbose N/A Administer anytime during HD Insulin Detemir, Isethionate (N/A) Administer anytime during HD Administer anytime during HD N/A and titrate, Administer anytime Insulin Glargine, 0.1-0.4mg PO Q8-12H, during HD Normal dose and titrate based on 100-150mg PO Q12-24H, <5 Insulin Lispro Acebutolol N/A Clonidine Administer anytime during HD; No target free or corrected total Administer post-HD (Low Flux) Phenytoin Dose post-HD if hypotensive 75-300mg PO Q24H, (Low Flux) phenytoin level, Normal dose based on indication, 0 Administer anytime during HD Acetaminophen N/A 75mg PO Q24H, Irbesartan Administer anytime during HD; Dose Administer anytime during HD Clopidogrel N/A (N/A) Administer anytime during HD post-HD if hypotensive No 15-45mg PO Q24H, Pioglitazone 2.5-5mg/kg IV/PO Q24H, (N/A) Administer anytime during HD 40-60 250-500mg PO Q6H or 1-2g IV Q4- 100mg IV weekly to monthly, Acyclovir Administer post-HD over 60 Cloxacillin N/A Iron Dextran N/A (N/A) 6H, Administer anytime -

Essential Medications Review
Essential medications for high-quality patient care Quarter 3 2021 update Essential medications – September 2021 As part of the mission to end drug shortages, the Vizient team of pharmacy experts continues to identify essential medications where, if not available, would prove the greatest threat to hospitals’ ability to provide immediate and high-quality patient care. Medications identified as of greatest importance were selected by the Vizient pharmacy team from a comprehensive clinical review of products contained within the World Health Organization’s (WHO) Essential Medicines list, the Advanced Cardiac Life Support (ACLS) and Pediatric Advanced Life Support (PALS) algorithms, and medications included in Vizient member health systems’ critical drug lists. As of this edition, 14 drugs were added to the list creating a total of 251 line items, representing 237 unique drugs and three categories. This includes: • Acute treatment drugs with no alternatives (77 drugs) – Medicines used in acute and critical circumstances to sustain life and for which there are no current alternatives • Chronic treatment drugs with no alternatives (37 drugs) – Products used in chronic disease states or conditions where no alternatives are available (e.g., chemotherapy medications) • High impact drugs (137 drugs) – Medicines for which alternatives are available but may be less clinically desirable and/or are more operationally difficult to use; also reflects drugs where the absence of one medication can affect therapeutically related drugs. Updated quarterly, -

Poison Control Antidote Poster
Antidote Chart (The suggested minimum stocking level is a combination of factors; anticipation of the highest total dose of a drug generally given during a 24 hour period to a 70 kg adult.) General Decontamination Uses Dose Comments Activated Charcoal (without sorbitol) Most ingestions occurring within one hour Adults: 25–100 grams, Children: 1 g/kg May be given in multiple doses depending on ingestant to enhance elimination Activated Charcoal (with sorbitol) May be used as first AC given to patient if presents within Adults: 25–50 grams Should not be used for multiple dose activated charcoal regimens one hour of ingestion Children: Not generally recommended Whole Bowel Irrigation Drugs not bound by charcoal, sustained Adults: 500–2000 ml/hour Nasogastric tube should be used to maintain amount given (Polyethylene Glycol) release formulations, and body stuffers Children: 25 ml/kg/hour Syrup of Ipecac No longer recommended No longer recommended No longer recommended Poisoning Antidote Quantity to Stock* Comments Acetaminophen N-ACETYLYCYSTEINE 10–20% 600 ml (20% Mucomyst®) Because vomiting of the oral NAC is common, the facility should maintain a repeat dose (Mucomyst®) or 1200 ml (10% Mucomyst®) for each patient for initial dosing if continuation of treatment at facility is not anticipated. ACETADOTE® The IV Acetadote® was just approved in February 2004. Call the MAPCC for dosing, ACETYLCYSTEINE Injection for IV use 4 (30 ml) vials of 20% solution precautions and contraindications. Both the oral and IV forms of acetylcysteine should be administered within 8 hours for maximal protection against hepatic injury. Anticholinergic Poisoning PHYSOSTIGMINE (Antilirium®) 2–4 mg* Not generally recommended for children. -

Toxic Exposure / Poisoning / Overdose
TOXIC EXPOSURE / POISONING / OVERDOSE GENERAL CONSIDERATIONS A. Consider the possibility of accidental or self-poisoning under the following conditions: 1. History of observed or admitted accidental or intentional ingestion 2. Coma of unknown origin 3. History of suicide gesture or attempt 4. Intoxicated behavior (hyperactive, hypoactive, unstable gait, lethargic, slurred speech) 5. Patients with altered mental status, particularly with bizarre behavior B. Scene safety is your primary concern. Potential threats include: 1. Violent, agitated behaviors from the patient 2. Possibility of the EMT becoming contaminated by the substance C. If suspected HazMat or WMD exposure: 1. Initial PPE should be Level A. 2. EMS personnel will NOT approach the victim without donning appropriate PPE or the victim has received emergency decontamination. D. Emergency decontamination procedures should be initiated whenever a victim has been exposed to a solid or liquid agent or the agent is unknown. With known agents, follow PPE and decontamination recommendations based on research. Emergency decontamination procedures should include: 1. Removal of all clothing, including undergarments 2. Body flushed with large quantities of water 3. Evacuation to an isolated area away from the agent release E. With known or suspected chemical / WMD exposure, contact Medical Control early and advise of the situation. F. EMS should consider the confirmed or potential release of a nerve agent when responding to an unspecified incident or scene involving: 1. An unknown illness involving a potentially large number of patients 2. An explosion from an unknown source at an event where a large number of people are in attendance 3. An incident where the initial EMS responders on scene suddenly become symptomatic 4. -

Chemical Compatibility Storage Group
CHEMICAL SEGREGATION Chemicals are to be segregated into 11 different categories depending on the compatibility of that chemical with other chemicals The Storage Groups are as follows: Group A – Compatible Organic Acids Group B – Compatible Pyrophoric & Water Reactive Materials Group C – Compatible Inorganic Bases Group D – Compatible Organic Acids Group E – Compatible Oxidizers including Peroxides Group F– Compatible Inorganic Acids not including Oxidizers or Combustible Group G – Not Intrinsically Reactive or Flammable or Combustible Group J* – Poison Compressed Gases Group K* – Compatible Explosive or other highly Unstable Material Group L – Non-Reactive Flammable and Combustible, including solvents Group X* – Incompatible with ALL other storage groups The following is a list of chemicals and their compatibility storage codes. This is not a complete list of chemicals, but is provided to give examples of each storage group: Storage Group A 94‐75‐7 2,4‐D (2,4‐Dichlorophenoxyacetic acid) 94‐82‐6 2,4‐DB 609-99-4 3,5-Dinitrosalicylic acid 64‐19‐7 Acetic acid (Flammable liquid @ 102°F avoid alcohols, Amines, ox agents see SDS) 631-61-8 Acetic acid, Ammonium salt (Ammonium acetate) 108-24-7 Acetic anhydride (Flammable liquid @102°F avoid alcohols see SDS) 79‐10‐7 Acrylic acid Peroxide Former 65‐85‐0 Benzoic acid 98‐07‐7 Benzotrichloride 98‐88‐4 Benzoyl chloride 107-92-6 Butyric Acid 115‐28‐6 Chlorendic acid 79‐11‐8 Chloroacetic acid 627‐11‐2 Chloroethyl chloroformate 77‐92‐9 Citric acid 5949-29-1 Citric acid monohydrate 57-00-1 Creatine 20624-25-3 -

Medical Uses of Sodium Thiosulfate Patrick L
McGeer PL, McGeer EG, Lee M. J Neurol Neuromedicine (2016) 1(3): 28-30 Neuromedicine www.jneurology.com www.jneurology.com Journal of Neurology & Neuromedicine Mini Review Open Access Medical uses of Sodium thiosulfate Patrick L. McGeer*, Edith G. McGeer and Moonhee Lee Kinsmen Laboratory of Neurological Research, University of British Columbia, VancouverBC V6T 1Z3, Canada Article Info Background Sodium thiosulfate (Na S O , STS) is an industrial compound Article Notes 2 2 3 Received: May 26, 2016 which has a long history of medical use. Since it is employed as a Accepted: June 21, 2016 food preservative, the general population is widely exposed to this *Correspondence: non-toxic compound. For example, it is typically added to table salt Dr. Patrick L. McGeer at less than 0.1% and to alcoholic beverages at less than 0.0005%. It Kinsmen Laboratory of Neurological Research, University of is generally available as a non-prescription oral product. British Columbia, 2255 Wesbrook Mall, Vancouver, BC V6T 1Z3 Canada One of the early medical uses of STS was in the successful Tel: +1-604-822-7377 1 FAX: +1-604-822-7086 treatment of arsenic, lead, mercury and bismuth poisoning . Halliday E-mail: [email protected] and Sutherland2 described using intravenous STS in a moribund © 2016 McGeer PL. This article is distributed under the terms of case of arsenic poisoning. There was an immediate response with the Creative Commons Attribution 4.0 International License eventual full recovery. Shiels3 described the use of STS to treat chronic lead poisoning at the Mount Ina mine in Australia.