Essential Medications Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
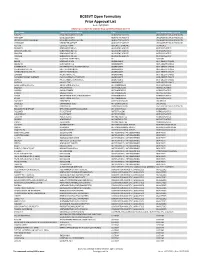
BCBSVT Open Formulary Prior Approval List
BCBSVT Open Formulary Prior Approval List As of: 10/27/2020 Helpful Tip: To search for a specific drug, use the find feature (Ctrl + F) Trade Name Chemical/Biological Name Class Prior Authorization Program FUSILEV LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS KHAPZORY LEVOLEUCOVORIN ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS LEVOLEUCOVORIN CALCIUM LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS VISTOGARD URIDINE TRIACETATE ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS ACTHAR CORTICOTROPIN ADRENAL HORMONES HORMONES BELRAPZO BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDAMUSTINE HCL BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDEKA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS TREANDA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS DAW (DISPENSE AS WRITTEN) ALL CUSTOM BELVIQ LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS BELVIQ XR LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS CONTRAVE ER NALTREXONE HCL/BUPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL ER DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS LOMAIRA PHENTERMINE HCL ANOREXIANTS ANTI‐OBESITY DRUGS PHENDIMETRAZINE TARTRATE PHENDIMETRAZINE TARTRATE ANOREXIANTS ANTI‐OBESITY DRUGS QSYMIA PHENTERMINE/TOPIRAMATE ANOREXIANTS ANTI‐OBESITY DRUGS SAXENDA LIRAGLUTIDE ANOREXIANTS ANTI‐OBESITY DRUGS ABIRATERONE ACETATE ABIRATERONE ACETATE ANTIANDROGENS ANTINEOPLASTICS ERLEADA APALUTAMIDE ANTIANDROGENS ANTINEOPLASTICS NUBEQA DAROLUTAMIDE ANTIANDROGENS -

Surfen, a Small Molecule Antagonist of Heparan Sulfate
Surfen, a small molecule antagonist of heparan sulfate Manuela Schuksz*†, Mark M. Fuster‡, Jillian R. Brown§, Brett E. Crawford§, David P. Ditto¶, Roger Lawrence*, Charles A. Glass§, Lianchun Wang*, Yitzhak Torʈ, and Jeffrey D. Esko*,** *Department of Cellular and Molecular Medicine, Glycobiology Research and Training Center, †Biomedical Sciences Graduate Program, ‡Department of Medicine, Division of Pulmonary and Critical Care Medicine and Veteran’s Administration San Diego Medical Center, ¶Moores Cancer Center, and ʈDepartment of Chemistry and Biochemistry, University of California at San Diego, La Jolla, CA 92093; and §Zacharon Pharmaceuticals, Inc, 505 Coast Blvd, South, La Jolla, CA 92037 Communicated by Carolyn R. Bertozzi, University of California, Berkeley, CA, June 18, 2008 (received for review May 26, 2007) In a search for small molecule antagonists of heparan sulfate, Surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide) was first we examined the activity of bis-2-methyl-4-amino-quinolyl-6- described in 1938 as an excipient for the production of depot carbamide, also known as surfen. Fluorescence-based titrations insulin (16). Subsequent studies have shown that surfen can indicated that surfen bound to glycosaminoglycans, and the extent block C5a receptor binding (17) and lethal factor (LF) produced of binding increased according to charge density in the order by anthrax (18). It was also reported to have modest heparin- heparin > dermatan sulfate > heparan sulfate > chondroitin neutralizing effects in an oral feeding experiments in rats (19), sulfate. All charged groups in heparin (N-sulfates, O-sulfates, and but to our knowledge, no further studies involving heparin have carboxyl groups) contributed to binding, consistent with the idea been conducted, and its effects on HS are completely unknown. -

2018-2019 Targeted Medication Safety Best Practices for Hospitals
2018-2019 Targeted Medication Safety Best Practices for Hospitals The purpose of the Targeted Medication Safety Best Practices for Hospitals is to identify, inspire, and mobilize widespread, national adoption of consensus-based best practices for specific medication safety issues that continue to cause fatal and harmful errors in patients, despite repeated warnings in ISMP publications. Hospitals can focus their medication safety efforts over the next 2 years on these best practices, which are realistic and have been successfully adopted by numerous organizations. While targeted for the hospital-based setting, some best practices may be applicable to other healthcare settings. The Targeted Medication Safety Best Practices for Hospitals have been reviewed by an external expert advisory panel and approved by the ISMP Board of Trustees. Related issues of the ISMP Medication Safety Alert! are referenced after each best practice. ISMP encourages hospitals that have not implemented the 2016-2017 Targeted Medication Safety Best Practices for Hospitals to do so as a priority, while implementing the 2018-2019 best practices. Organizations need to focus on previous best practices 2, 3, 9 and 11 since these have the lowest implementation rate. Two of the 2016-2017 Targeted Medication Safety Best Practices for Hospitals (number 4 and 7) have been revised for 2018-2019. Best practices number 12 through 14 are new for 2018-2019. www.ismp.org BEST PRACTICE 1: Dispense vinCRIStine (and other vinca alkaloids) in a minibag of a compatible solution and not in a syringe. Rationale: Related ISMP Medication The goal of this best practice is to ensure that vinca alkaloids are Safety Alerts!: administered by the intravenous route only. -

Revista Brasileira De Anestesiologia
Rev BrasBras Anestesiol. Anestesiol. 2013;63(1):163–164 2013;63(1):163-166 REVISTA BRASILEIRA DE ANESTESIOLOGIA Offi cial Publication of the Brazilian Society of Anesthesiology www.sba.com.br/rba/index.asp LETTER TO THE EDITOR Precipitation in Gallipoli: Sugammadex / Amiodarone & Sugammadex / Dobutamine & Sugammadex / Protamine Dear Editor, furosemide (10 mg.mL-1), gentamicin (40 mg.mL-1), glyceryl Sugammadex is a modifi ed gamma cyclodextrin 1-3. Cyclodextrins trinitrate (5 mg.mL-1), heparin (1,000 IU.mL-1), hydrocor- are water soluble cyclic oligosaccharides with a lipophilic tisone (250 mg.mL-1), crystallized insulin (100 IU.mL-1), core. Sugammadex has quickly found a place in clinical use Calcium (Calcium Gluconate Monohydrate 225 mg.10 mL-1 for selective antagonism of neuromuscular blockade with ro- + Calcium levulinate dihitrate 572 mg.10 mL-1), ketamine curonium 1-3. Sugammadex quickly encapsulates steroidal neu- (50 mg.mL-1), levobupivacaine (7.5 mg.mL-1), magnesium romuscular blockers, increasing the amount of encapsulated sulphate (1.2 mEq.mL-1), metamizol sodium (0.5 g.mL-1), steroidal neuromuscular blockers in plasma and separating the methylergobasin maleate (0.2 mg.mL-1), metoclopramide blockers from the nicotinic acetylcholine receptors 1-3. (5 mg.mL-1), metoprolol (1 mg.mL-1), morphine (0.01 g.mL-1), Apart from its use with steroidal neuromuscular block- midazolam (5 mg.mL-1), n-acetylcysteine (100 mg.mL-1), ers, it is known that sugammadex interacts with over 40 naloxone (0.4 mg.mL-1), neostigmine (0.5 mg.mL-1), nitroprus- lipophilic, steroidal and non-steroidal drugs. -

Thiosulfate De Sodium Sterop
Thiosulfate de Sodium Sterop 1. DENOMINATION DU MEDICAMENT THIOSULFATE DE SODIUM STEROP 1g/5ml solution injectable 2. COMPOSITION QUALITATIVE ET QUANTITATIVE Substance active : Une ampoule de 5ml contient 1g de thiosulfate de sodium dans de l’eau pour préparations injectables. 1 ml de solution contient 200mg de thiosulfate de sodium. Pour la liste complète des excipients, voir rubrique 6.1. 3. FORME PHARMACEUTIQUE Solution injectable. 4. DONNEES CLINIQUES 4.1 Indications thérapeutiques - Antidote dans le traitement des intoxications par les cyanures ainsi que par le nitroprussiate de sodium. - Prévention des effets néphrotoxiques du cisplatine. Thiosulfate de Sodium Sterop © Pharma.be Pagina 1 van 6 4.2 Posologie et mode d’administration Mode d'administration Pour voie intraveineuse lente. Posologie Intoxications par les cyanures: Adultes: La posologie habituelle est de 12,5 g de thiosulfate de sodium, administré par voie I.V. sur une période de 5 à 10 minutes. Enfants: La posologie habituelle est de 412,5 mg de thiosulfate de sodium par kg, administré par voie I.V. à la vitesse de 0,625 à 1,25 g/minute sur une période de 5 à 10 minutes. Si les symptômes persistent ou réapparaissent dans la demi-heure ou dans l’heure ayant suivi la première injection, une seconde injection de thiosulfate de sodium est nécessaire avec comme posologie la moitié de celle de la première injection. En prévention des effets néphrotoxiques du cisplatine: Chez l'adulte, la posologie initiale est de 9 g de thiosulfate de sodium par m2 de surface corporelle, administré par voie I.V. Elle est suivie par l'injection de 1,2 g de thiosulfate de sodium par m2 de surface corporelle par heure, en perfusion I.V. -
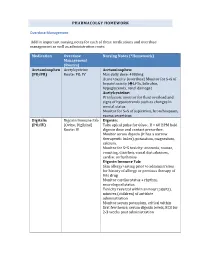
PHARMACOLGY HOMEWORK Overdose Management Add In
PHARMACOLGY HOMEWORK Overdose Management Add in important nursing notes for each of these medications and overdose management as well as administration route. Medication Overdose Nursing Notes (*Homework) Management (Routes) Acetaminophen Acetylcysteine Acetaminophen: (PO/PR) Route: PO, IV Max daily dose: 4000mg Acute toxicity (overdose) Monitor for S+S of hepatotoxicity (éLFTs, bilirubin, hypoglycemia, renal damage) Acetylcysteine: IV infusion: monitor for fluid overload and signs of hyponatremia such as changes in mental status Monitor for S+S of aspiration, bronchospasm, excess secretions Digitalis Digoxin Immune Fab Digoxin: (PO/IV) (Ovine, Digibind) Take apical pulse for 60sec. If < 60 BPM hold Route: IV digoxin dose and contact prescriber. Monitor serum digoxin (it has a narrow therapeutic index), potassium, magnesium, calcium. Monitor for S+S toxicity: anorexia, nausea, vomiting, diarrhea, visual disturbances, cardiac arrhythmias Digoxin Immune Fab: Skin allergy testing prior to administration for history of allergy or previous therapy of this drug Monitor cardiac status + rhythm, neurological status Toxicity reversal within an hour (adults), minutes (children) of antidote administration Monitor serum potassium, critical within first few hours; serum digoxin levels, ECG for 2-3 weeks post administration Heparin Protamine sulfate Heparin: (IV/SC) (IV) Monitor for spontaneous bleeding, thrombocytopenia Monitor aPTT levels Protamine Sulfate: Sudden drop in BP Monitor BP+ P q15-30 min Monitor aPTT Opioid Naloxone (IV-adults, Opioids: -

Wednesday, February 10, 2016 4 P.M
Wednesday, February 10, 2016 4 p.m. Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review (DUR) Board Members FROM: Bethany Holderread, Pharm.D. SUBJECT: Packet Contents for DUR Board Meeting – February 10, 2016 DATE: February 1, 2016 Note: The DUR Board will meet at 4:00p.m. The meeting will be held at 4345 N Lincoln Blvd. Enclosed are the following items related to the February meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/Oral Viscous Lidocaine Claims Analysis Update – Appendix B Action Item – Vote to Prior Authorize Duopa™ (Carbidopa/Levodopa Enteral Suspension) and Rytary™ (Carbidopa/Levodopa Extended-Release Capsules) – Appendix C Action Item – Vote to Prior Authorize Cortisporin® and Pediotic® (Neomycin/Polymyxin B/Hydrocortisone Otic) – Appendix D Action Item – Vote to Prior Authorize Migranal® (Dihydroergotamine Nasal Spray) – Appendix E Action Item – Vote to Prior Authorize Strensiq™ (Asfotase Alfa) – Appendix F Action Item – Vote to Prior Authorize Varubi™ (Rolapitant) – Appendix G Action Item – Vote to Prior Authorize Xuriden™ (Uridine Triacetate) – Appendix H Annual Review of Gout Medications and 30-Day Notice to Prior Authorize Mitigare™ (Colchicine Capsules) and Zurampic® (Lesinurad) – Appendix I Annual Review of Seizure Medications and 30-Day Notice to Prior Authorize Spritam® (Levetiracetam) – Appendix J 30-Day Notice to Prior Authorize Solaraze® (Diclofenac Gel) – Appendix K Annual Review of Ulcerative Colitis Medications and 30-Day Notice to Prior Authorize Uceris® (Budesonide Extended-Release Tablets), Uceris® (Budesonide Rectal Foam), and Miscellaneous Mesalamine Products – Appendix L Annual Review of Ocular Allergy Medications and 30-Day Notice to Prior Authorize Pazeo® (Olopatadine Ophthalmic) – Appendix M ORI-4403 • P.O. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Database for Drug Metabolism and Comparisons, Nicedrug.Ch, Aids
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.28.120782; this version posted June 30, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Database for drug metabolism and comparisons, NICEdrug.ch, aids discovery and design 2 3 Authors/affiliation 1 1,2,5 1,3,5 4 Homa MohammadiPeyhani , Anush Chiappino-Pepe , Kiandokht Haddadi , Jasmin 1 1,4 1,* 5 Hafner , Noushin Hadadi , Vassily Hatzimanikatis 6 1 7 Laboratory of Computational Systems Biotechnology, École Polytechnique Fédérale de 8 Lausanne, EPFL, Lausanne, Switzerland 2 9 Present address: Department of Genetics, Harvard Medical School, Boston, Massachusetts, 10 USA 3 11 Present address: Department of Chemical Engineering and Applied Chemistry, University of 12 Toronto, Toronto, Canada 4 13 Present address: Department of Cell Physiology and Metabolism, Université de Genève, 14 Geneva, Switzerland 5 15 These authors contributed equally 16 * 17 Corresponding author: 18 Prof. Vassily Hatzimanikatis 19 Laboratory of Computational Systems Biotechnology (LCSB), École Polytechnique Fédérale de 20 Lausanne (EPFL), CH‐1015 21 Lausanne, Switzerland 22 Email: [email protected] , Phone: +41 (0)21 693 98 70, Fax: +41 (0)21 693 98 75 23 24 Abstract 25 The discovery of a drug requires over a decade of enormous research and financial 26 investments—and still has a high risk of failure. To reduce this burden, we developed the 27 NICEdrug.ch database, which incorporates 250,000 bio-active molecules, and studied their 28 metabolic targets, fate, and toxicity. -

Protamine Characterization by Top-Down Proteomics: Boosting Proteoform Identification with DBSCAN
proteomes Article Protamine Characterization by Top-Down Proteomics: Boosting Proteoform Identification with DBSCAN Gianluca Arauz-Garofalo 1, Meritxell Jodar 2,3 , Mar Vilanova 1, Alberto de la Iglesia Rodriguez 2, Judit Castillo 2 , Ada Soler-Ventura 2, Rafael Oliva 2,3,* , Marta Vilaseca 1,* and Marina Gay 1,* 1 Institute for Research in Biomedicine (IRB Barcelona), The Barcelona Institute of Science and Technology, Baldiri Reixac, 10, 08028 Barcelona, Spain; [email protected] (G.A.-G.); [email protected] (M.V.) 2 Molecular Biology of Reproduction and Development Research Group, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Fundació Clínic per a la Recerca Biomèdica (FCRB), Faculty of Medicine and Health Sciences, University of Barcelona, 08036 Barcelona, Spain; [email protected] (M.J.); [email protected] (A.d.l.I.R.); [email protected] (J.C.); [email protected] (A.S.-V.) 3 Biochemistry and Molecular Genetics Service, Hospital Clínic de Barcelona, 08036 Barcelona, Spain * Correspondence: [email protected] (R.O.); [email protected] (M.V.); [email protected] (M.G.) Abstract: Protamines replace histones as the main nuclear protein in the sperm cells of many species and play a crucial role in compacting the paternal genome. Human spermatozoa contain protamine 1 (P1) and the family of protamine 2 (P2) proteins. Alterations in protamine PTMs or the P1/P2 ratio Citation: Arauz-Garofalo, G.; may be associated with male infertility. Top-down proteomics enables large-scale analysis of intact Jodar, M.; Vilanova, M.; proteoforms derived from alternative splicing, missense or nonsense genetic variants or PTMs. -

New Drug Approvals and Extended Indications for Infants, Children, and Adolescents Marcia L
PEDIATRIC PHARMACOTHERAPY Volume 21 Number 11 November 2015 New Drug Approvals and Extended Indications for Infants, Children, and Adolescents Marcia L. Buck, PharmD, FCCP, FPPAG ver the past six months, a number of which slowly releases the drug over time, O significant new drugs have been approved providing up to 13 hours of symptom control. by the Food and Drug Administration (FDA). In The safety and efficacy of the product was addition, several drugs already on the market established in a phase 3 randomized, placebo- have been granted an indication for use in controlled trial in 108 children with ADHD.4 pediatric patients. Following a 5-week open-label dose optimization period, patients were randomized to New Drug Product Approvals treatment (2.5-10 mg) or placebo for a 1-week period. At the end of the week, scores on the Adapalene and Benzoyl Peroxide Swanson, Kotkin, Agler, M-Flynn, and Pelham Epiduo Forte®, a new product containing (SKAMP)-Combined rating scale were adapalene 0.3%, a retinoid, and benzoyl peroxide compared to baseline. The change in scores after 2.5% was approved on July 16, 2015 for the treatment demonstrated a statistically significant treatment of moderate to severe acne in adults improvement throughout the day compared to and children 12 years of age and older.1 The placebo (assessed at 1, 2, 6, 8, 10, 12, and 13 efficacy of the new product was demonstrated in hours post-dose), with a mean change of -8.8 (SE a phase 3 multicenter randomized, double-blind 1.14) in the treated patients and 6.0 (SE 1.19) in trial comparing it to the gel vehicle without the the controls. -

Adult BLS Standing Orders • Ensure EMS Provider Safety, Consider HAZMAT Activation
Imperial County Public Health Department Emergency Medical Services Agency Policy/Procedure/Protocol Manual Treatment Protocols Date: 07/01/2021 Poisoning/Intoxication/Envenomation - Adult Policy #9160A Adult BLS Standing Orders • Ensure EMS provider safety, consider HAZMAT activation. Recognize, Notify, Isolate • Universal Patient Protocol • Do not approach patient or location if scene safety is in question • Obtain accurate history of incident: o Name of product or substance o Quantity ingested, and/or duration of exposure o Time elapsed since exposure o If safe and accessible, bring medications or bottles to hospital • Move victim(s) to safe environment • Externally decontaminate - PRN • Continuously monitor ECG, blood pressure, pulse oximetry, and capnography (if ALS present) PRN • Give oxygen and provide airway support per Airway Policy • Contact Poison Control Center as needed 1 (800) 222-1222 Suspected Opioid Overdose with Respirations <12 RPM • If possible, avoid the use of a supraglottic device prior to the administration of naloxone • Administer naloxone 0.1 mg/kg, max of 2 mg IN. May repeat up to three (3) times, q5min • May assist family/friends on-scene with administration of patient’s own naloxone • NOTE - Use with caution in opioid dependent pain management patients • Assess vitals, with specific attention to respiratory rate and respiratory drive • Note pupil exam • Note drug paraphernalia or medication bottles near patient Suspected Stimulant Overdose with Sudden Hypoventilation, Oxygen Desaturation, or Apnea • High flow