Antidote Stocking Tier C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Management of Poisoning
MOH CLINICAL PRACTICE GUIDELINES December/2011 Management of Poisoning Health Ministry of Sciences Chapter of Emergency College of College of Family Manpower Authority Physicians Physicians, Physicians Academy of Medicine, Singapore Singapore Singapore Singapore Medical Pharmaceutical Society Society for Emergency Toxicology Singapore Paediatric Association of Singapore Medicine in Singapore Society (Singapore) Society Executive summary of recommendations Details of recommendations can be found in the main text at the pages indicated. Principles of management of acute poisoning – resuscitating the poisoned patient GPP In a critically poisoned patient, measures beyond standard resuscitative protocol like those listed above need to be implemented and a specialist experienced in poisoning management should be consulted (pg 55). GPP D Prolonged resuscitation should be attempted in drug-induced cardiac arrest (pg 55). Grade D, Level 3 1 C Titrated doses of naloxone, together with bag-valve-mask ventilation, should be administered for suspected opioid-induced coma, prior to intubation for respiratory insuffi ciency (pg 56). Grade C, Level 2+ D In bradycardia due to calcium channel or beta-blocker toxicity that is refractory to conventional vasopressor therapy, intravenous calcium, glucagon or insulin should be used (pg 57). Grade D, Level 3 B Patients with actual or potential life threatening cardiac arrhythmia, hyperkalaemia or rapidly progressive toxicity from digoxin poisoning should be treated with digoxin-specifi c antibodies (pg 57). Grade B, Level 2++ B Titrated doses of benzodiazepine should be given in hyperadrenergic- induced tachycardia states resulting from poisoning (pg 57). Grade B, Level 1+ D Non-selective beta-blockers, like propranolol, should be avoided in stimulant toxicity as unopposed alpha agonism may worsen accompanying hypertension (pg 57). -

Cyanide Poisoning and How to Treat It Using CYANOKIT (Hydroxocobalamin for Injection) 5G
Cyanide Poisoning and How to Treat It Using CYANOKIT (hydroxocobalamin for injection) 5g 1. CYANOKIT (single 5-g vial) [package insert]. Columbia, MD: Meridian Medical Technologies, Inc.; 2011. Please see Important Safety Information on slides 3-4 and full Prescribing Information for CYANOKIT starting on slide 33. CYANOKIT is a registered trademark of SERB Sarl, licensed by Meridian Medical Technologies, Inc., a Pfizer company. Copyright © 2015 Meridian Medical Technologies, Inc., a Pfizer company. All rights reserved. CYK783109-01 November/2015. Indication and Important Safety Information……………………………………………………………………………….………..…..3 . Identifying Cyanide Poisoning……………………………………………………………………………………………………………….…………….….5 . How CYANOKIT (hydroxocobalamin for injection) Works……………………………………………………………….12 . The Specifics of CYANOKIT…………………………………………………………………………………………………………………………….………17 . Administering CYANOKIT………………………………………………………………………………………………………………………………..……….21 . Storage and Disposal of CYANOKIT…................................................................................................................................26 . Grant Information for CYANOKIT……………………………………………………………………………………………………………………....30 . Full Prescribing Information………………………………………………………………………………………………….………………………………33 Please see Important Safety Information on slides 3-4 and full Prescribing Information for CYANOKIT starting on slide 33. CYANOKIT (hydroxocobalamin for injection) 5 g for intravenous infusion is indicated for the treatment of known or suspected cyanide poisoning. -

Evaluation of Neuropharmacological Activity of Hydroalcoholic Extract of Fruits of Trapa Bispinosa in Laboratory Animals
International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 2, Suppl 2, 2010 Research Article EVALUATION OF NEUROPHARMACOLOGICAL ACTIVITY OF HYDROALCOHOLIC EXTRACT OF FRUITS OF TRAPA BISPINOSA IN LABORATORY ANIMALS VYAWAHARE N. S.a AND AMBIKAR D. B.* b aDepartment of Pharmacology, AISSMS College of Pharmacy, Kennedy Road, Pune 411 001, India. bDepartment of Pharmacology, Marathwada Mitra Mandals College of Pharmacy, Thergaon, Pune 411033, India. Email: [email protected] Received: 26 Dec 2009, Revised and Accepted:19 Jan 2010 ABSTRACT The present investigation deals with evaluation of neuropharmacological activity of different doses (100, 250, 500mg/kg, p.o.) of hydroalcoholic extract of Trapa bispinosa (TB) in laboratory animals. The effects of extract on various parameters like motor coordination, spontaneous locomotor activity, object recognition, transfer latency, anxiolytic activity, analgesic activity, sodium nitrite induced respiratory arrest and hypoxic stress etc was studied. The TB (250 & 500mg/kg) found to decrease time required to occupy the central platform (transfer latency) in the elevated plus maze and to increase discrimination index in the object recognition test, indicating nootropic activity. TB (250 and 500mg/kg) showed significant increase in reaction time in hot plate analgesic activity. Moreover it also showed significant reduction in spontaneous locomotor activity and latency to death in sodium nitrite induced respiratory arrest. To conclude hydroalcoholic extract of TB possesses significant facilitation of learning and memory which may be due to enhanced cholinergic function. TB also showed significant analgesic activity. Key words: Trapa bispinosa, Nootropic, Analgesic. Chemicals and drugs INTRODUCTION Sodium nitrate was purchased from Loba chemicals, Mumbai. Medicinal herbs constitute the cornerstone of traditional medicinal Diazepam injection, pentazocin injection, piracetam syrup, was practice worldwide. -

Letter to the Editor Effect of N-Acetylcysteine And
Indian J Physiol Pharmacol 1999; 43 (4): 518-520 LETTER TO THE EDITOR EFFECT OF N-ACETYLCYSTEINE AND SODIUM NITRITE ON MYOCARDIAL REPERFUSION INJURY Sir, (Received on May 22, 1999) Results of experimental studies suggest India). Heart was suspended in pericardial the involvement of oxygen free radicals cradle after left sided thoracotomy at fourth (OFR) in myocardial reperfusion injury 0, intercostal space. A polythene catheter 2). Ischemia is known to deplete endogenous (1.5 mm inner diameter) was placed in the antioxidants like superoxide dismutase, left ventricle through apex t~ [ecord glutathione, catalase 0, 2) and makes ventricular pressure changes on Grass 16 myocardial, cell vulnerable to oxygen free channel polygraph (Model 79D, USA) by radical (OFR) induced damage 0, 2). OFR using Gould pressure transducer. Heart rate can damage endothelial cells at the time of and ST segment changes were monitored in reperfusion (3). Endothelial cells synthesise standard limb leads on BPL (India) and form nitric oxide which is endothelium, electrocardiograph. Another two catheters derived relaxation factor (4). Acidified were placed in the left atrium for infusion sodium nitrite (Na Oz) at pH-2 forms nitric of saline, drug or acidified N aNOz' Left oxide (5) which can inactivate superoxide anterior descending (LAD) coronary artery radicals (5, 6). N-acetylcysteine (NAC) is was dissected free above first diagonal known to replenish glutathione stores, branch and below the origin of left increase superoxide dismutase activity, circumflex artery. Duration of LAD coronary scanvenge hydroxyl radicals and interfere artery occlusion was 90-min. Following with autocatalytic lipid peroxidation (7, 8). completion of occlusion, reperfusion of the In our previous studies we have shown the myocardium was initiated by removing the individual effects of NAC and NaNOz on vessel occludeI'. -

Long-Term N-Acetylcysteine and L-Arginine Administration Reduces Endothelial Activation and Systolic Blood Pressure in Hypertensive Patients with Type 2 Diabetes
Emerging Treatments and Technologies ORIGINAL ARTICLE Long-Term N-Acetylcysteine and L-Arginine Administration Reduces Endothelial Activation and Systolic Blood Pressure in Hypertensive Patients With Type 2 Diabetes 1 1 VALENTINO MARTINA, MD PAOLA MASSARENTI, MD ardiovascular complications repre- 1 1 ANDI MASHA, MD FABIO SETTANNI, PHD sent 80% of the causes of death in 1 2 VALENTINA RAMELLA GIGLIARDI, MD LARA DELLA CASA, PHD 1 patients with type 2 diabetes. Several OREDANA ROCATO MD 2 C L B , STEFANIA BERGAMINI, PHD causes may explain this mortality excess. 3 2 ENZO MANZATO, MD, PHD NNA ANNONE MD, PHD 1 A I , Among these, the decreased availability of ARRIGO BERCHIO, MD nitric oxide (NO) has increasingly gained credit. In fact, reduced NO availability has been demonstrated not only in diabe- OBJECTIVE — Reactive oxygen and nitric oxide (NO) have recently been considered to be tes (1) but also in other diseases, such as involved in the cardiovascular complications of patients with type 2 diabetes, as NO is thought atherosclerosis and hypertension, known to lose its beneficial physiological effects in the presence of oxygen radicals. For this reason, we to be associated with increased mortality tested the effects of L-arginine (ARG) and N-acetylcysteine (NAC) administration in increasing due to cardiovascular causes (2,3). NO is NO bioavailability by reducing free radical formation. crucial for regulating the vascular tone RESEARCH DESIGN AND METHODS — A double-blind study was performed on 24 and maintaining the intrinsic thrombore- male patients with type 2 diabetes and hypertension divided into two groups of 12 patients that sistant and atheroprotective properties of randomly received either an oral supplementation of placebo or NAC ϩ ARG for 6 months. -

GHS Calcium Lactate Gluconate MSDS.Pdf
Safety Data Sheet (Calcium Lactate Gluconate) DATE PREPARED: 7/9/2015 Section 1. Product and Company Identification Product Name Calcium Lactate Gluconate CAS Number 11116-97-5 Parchem - fine & specialty chemicals EMERGENCY RESPONSE NUMBER 415 Huguenot Street CHEMTEL New Rochelle, NY 10801 Toll Free US & Canada: 1 (800) 255-3924 (914) 654-6800 (914) 654-6899 All other Origins: 1 (813) 248-0585 parchem.com [email protected] Collect Calls Accepted Section 2. Hazards Identification Classification of the substance or mixture Classification according to Directive 67/548/EEC or 1999/45/EC as amended: This preparation does not meet the criteria for classification according to Directive 1999/45/EC as amended. Classification according to Regulation (EC) No 1272/2008 as amended: This mixture does not meet the criteria for classification according to Regulation (EC) 1272/2008 as amended. Hazard and precautionary statements Hazard statements: The substance does not meet the criteria for classification. Precautionary statements: Not available. Appearance & Odor: White powder with no odor. Fire & Explosion Hazards Potential for dust explosion may exist. This product is not defined as flammable or combustible. However, the product may decompose under fire conditions to produce toxic oxides of carbon. Depending upon conditions, dusts may be sensitive to static discharge. Avoid possibility of dry powder and friction causing static electricity in presence of flammables (See NFPA-77, Chpt. 6) Primary Route of Exposure: Skin and eye contact are the primary routes of exposure to this product. Inhalation Acute Exposure: Inhalation of dust may cause mild irritation. Skin Contact - Acute: Skin contact is not expected to cause irritation. -

Uses and Administration Adverse Effects and Precautions Pharmacokinetics Uses and Administration
1549 The adverse effects of dicobalt edetate are more severe in year-old child: case report and review of literature. Z Kardiol 2005; 94: References. 817-2 3. the absence of cyanide. Therefore, dicobalt edetate should I. Schaumann W, et a!. Kinetics of the Fab fragments of digoxin antibodies and of bound digoxin in patients with severe digoxin intoxication. Eur 1 not be given unless cyanide poisoning is confirmed and 30: Administration in renal impairment. In renal impairment, Clin Pharmacol 1986; 527-33. poisoning is severe such as when consciousness is impaired. 2. Ujhelyi MR, Robert S. Pharmacokinetic aspects of digoxin-specific Fab elimination of antibody-bound digoxin or digitoxin is therapy in the management of digitalis toxicity. Clin Pharmacokinet 1995; Oedema. A patient with cyanide toxicity developed delayed1·3 and antibody fragments can be detected in the 28: 483-93. plasma for 2 to 3 weeks after treatment.1 The rebound in 3. Renard C, et al. Pharmacokinetics of dig,'><i<1-sp,eei1ie Fab: effects of severe facial and pulmonary oedema after treatment with decreased renal function and age. 1997; 44: 135-8. dicobalt edetate.1 It has been suggested that when dicobalt free-digoxin concentrations that has been reported after edetate is used, facilities for intubation and resuscitation treatment with digoxin-specific antibody fragments (see P (Jrations should be immediately available. Poisoning, below), occurred much later in patients with r.�p renal impairment than in those with normal renal func Proprietary Preparations (details are given in Volume B) 1. Dodds C, McKnight C. Cyanide toxicity after immersion and the hazards tion. -

Wednesday May 26, 1999
5±26±99 Vol. 64 No. 101 Wednesday Pages 28333±28712 May 26, 1999 federal register 1 VerDate 06-MAY-99 21:29 May 25, 1999 Jkt 183247 PO 00000 Frm 00001 Fmt 4710 Sfmt 4710 E:\FR\FM\26MYWS.XXX pfrm03 PsN: 26MYWS II Federal Register / Vol. 64, No. 101 / Wednesday, May 26, 1999 The FEDERAL REGISTER is published daily, Monday through SUBSCRIPTIONS AND COPIES Friday, except official holidays, by the Office of the Federal Register, National Archives and Records Administration, PUBLIC Washington, DC 20408, under the Federal Register Act (44 U.S.C. Subscriptions: Ch. 15) and the regulations of the Administrative Committee of Paper or fiche 202±512±1800 the Federal Register (1 CFR Ch. I). The Superintendent of Assistance with public subscriptions 512±1806 Documents, U.S. Government Printing Office, Washington, DC 20402 is the exclusive distributor of the official edition. General online information 202±512±1530; 1±888±293±6498 Single copies/back copies: The Federal Register provides a uniform system for making available to the public regulations and legal notices issued by Paper or fiche 512±1800 Federal agencies. These include Presidential proclamations and Assistance with public single copies 512±1803 Executive Orders, Federal agency documents having general FEDERAL AGENCIES applicability and legal effect, documents required to be published Subscriptions: by act of Congress, and other Federal agency documents of public Paper or fiche 523±5243 interest. Assistance with Federal agency subscriptions 523±5243 Documents are on file for public inspection in the Office of the Federal Register the day before they are published, unless the issuing agency requests earlier filing. -

Sodium Nitrite and Sodium Thiosulfate
PATIENT & CAREGIVER EDUCATION Sodium Nitrite and Sodium Thiosulfate This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Nithiodote Warning This drug may cause low blood pressure and a red blood cell problem called methemoglobinemia. These may be life-threatening. This drug is only for use when cyanide poisoning is life-threatening. This drug must be used with care if it is not known if cyanide poisoning has happened. Talk with the doctor. Tell the doctor if your child has inhaled a lot of smoke or if your child has any of these health problems: Anemia, heart problems, lack of a certain enzyme called congenital methemoglobin reductase deficiency, or lung problems. What is this drug used for? It is used to treat cyanide poisoning. What do I need to tell the doctor BEFORE my child takes this drug? If your child is allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell the doctor about the allergy and what signs your child had. Sodium Nitrite and Sodium Thiosulfate 1/6 If your child is breast-feeding a baby: Be sure your child does not breast-feed a baby while taking this drug. This drug may interact with other drugs or health problems. Tell the doctor and pharmacist about all of your child’s drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe to give this drug with all of your child’s other drugs and health problems. -

Thiosulfate De Sodium Sterop
Thiosulfate de Sodium Sterop 1. DENOMINATION DU MEDICAMENT THIOSULFATE DE SODIUM STEROP 1g/5ml solution injectable 2. COMPOSITION QUALITATIVE ET QUANTITATIVE Substance active : Une ampoule de 5ml contient 1g de thiosulfate de sodium dans de l’eau pour préparations injectables. 1 ml de solution contient 200mg de thiosulfate de sodium. Pour la liste complète des excipients, voir rubrique 6.1. 3. FORME PHARMACEUTIQUE Solution injectable. 4. DONNEES CLINIQUES 4.1 Indications thérapeutiques - Antidote dans le traitement des intoxications par les cyanures ainsi que par le nitroprussiate de sodium. - Prévention des effets néphrotoxiques du cisplatine. Thiosulfate de Sodium Sterop © Pharma.be Pagina 1 van 6 4.2 Posologie et mode d’administration Mode d'administration Pour voie intraveineuse lente. Posologie Intoxications par les cyanures: Adultes: La posologie habituelle est de 12,5 g de thiosulfate de sodium, administré par voie I.V. sur une période de 5 à 10 minutes. Enfants: La posologie habituelle est de 412,5 mg de thiosulfate de sodium par kg, administré par voie I.V. à la vitesse de 0,625 à 1,25 g/minute sur une période de 5 à 10 minutes. Si les symptômes persistent ou réapparaissent dans la demi-heure ou dans l’heure ayant suivi la première injection, une seconde injection de thiosulfate de sodium est nécessaire avec comme posologie la moitié de celle de la première injection. En prévention des effets néphrotoxiques du cisplatine: Chez l'adulte, la posologie initiale est de 9 g de thiosulfate de sodium par m2 de surface corporelle, administré par voie I.V. Elle est suivie par l'injection de 1,2 g de thiosulfate de sodium par m2 de surface corporelle par heure, en perfusion I.V. -
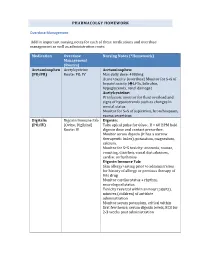
PHARMACOLGY HOMEWORK Overdose Management Add In
PHARMACOLGY HOMEWORK Overdose Management Add in important nursing notes for each of these medications and overdose management as well as administration route. Medication Overdose Nursing Notes (*Homework) Management (Routes) Acetaminophen Acetylcysteine Acetaminophen: (PO/PR) Route: PO, IV Max daily dose: 4000mg Acute toxicity (overdose) Monitor for S+S of hepatotoxicity (éLFTs, bilirubin, hypoglycemia, renal damage) Acetylcysteine: IV infusion: monitor for fluid overload and signs of hyponatremia such as changes in mental status Monitor for S+S of aspiration, bronchospasm, excess secretions Digitalis Digoxin Immune Fab Digoxin: (PO/IV) (Ovine, Digibind) Take apical pulse for 60sec. If < 60 BPM hold Route: IV digoxin dose and contact prescriber. Monitor serum digoxin (it has a narrow therapeutic index), potassium, magnesium, calcium. Monitor for S+S toxicity: anorexia, nausea, vomiting, diarrhea, visual disturbances, cardiac arrhythmias Digoxin Immune Fab: Skin allergy testing prior to administration for history of allergy or previous therapy of this drug Monitor cardiac status + rhythm, neurological status Toxicity reversal within an hour (adults), minutes (children) of antidote administration Monitor serum potassium, critical within first few hours; serum digoxin levels, ECG for 2-3 weeks post administration Heparin Protamine sulfate Heparin: (IV/SC) (IV) Monitor for spontaneous bleeding, thrombocytopenia Monitor aPTT levels Protamine Sulfate: Sudden drop in BP Monitor BP+ P q15-30 min Monitor aPTT Opioid Naloxone (IV-adults, Opioids: -

Calcium Gluconate
CALCIUM GLUCONATE CLASSIFICATION Minerals and electrolytes TRADE NAME(S) Calcium Gluconate 10% (for IV use) Calcium Gluconate gel 2.5% (for topical use) DESIRED EFFECTS Lower potassium levels; pain relief and neutralizing fluoride ion MECHANISM OF ACTION Calcium is the primary component of skeletal tissue. Bone serves as a calcium depot and as a reservoir for electrolytes and buffers. INDICATIONS Suspected hyperkalemia in adult PEA/Asystole Antidote for calcium channel blocker overdose and magnesium sulfate toxicity Gel is used for hydrofluoric acid burns Suspected hyperkalemia with adult crush injury or peaked T-waves on EKG CONTRAINDICATIONS Should not be given to patients with digitalis toxicity Should be used with caution in patients with dehydration ADVERSE REACTIONS When given too rapidly or to someone on digitalis, can cause sudden death from ventricular fibrillation May cause mild to severe IV site irritation SPECIAL CONSIDERATIONS Must either use a different IV line or flush line with copious normal saline if being given with sodium bicarbonate. When used on hydroflouric acid burns, relief of pain is the only indication of treatment efficacy. Therefore, use of analgesic agents is not recommended. DOSING REGIMEN Suspected hyperkalemia in adult PEA/asystole or adult crush injury, or evidence of EKG changes (Ex. peaked T-waves) o Calcium gluconate 10% 15-30 ml IV/IO over 2-5 minutes KNOWN calcium channel blocker overdose - administer 3 grams IV/IO may repeat dose in 10 minutes if no effect. Hydrofluoric acid burns apply calcium gluconate gel 2.5% every 15 minutes to burned area and massage continuously until pain disappears.