Uses and Administration Adverse Effects and Precautions Pharmacokinetics Uses and Administration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review of Succimer for Treatment of Lead Poisoning
Review of Succimer for treatment of lead poisoning Glyn N Volans MD, BSc, FRCP. Department of Clinical Pharmacology, School of Medicine at Guy's, King's College & St Thomas' Hospitals, St Thomas' Hospital, London, UK Lakshman Karalliedde MB BS, DA, FRCA Consultant Medical Toxicologist, CHaPD (London), Health Protection Agency UK, Visiting Senior Lecturer, Division of Public Health Sciences, King's College Medical School, King's College , London Senior Research Collaborator, South Asian Clinical Toxicology Research Collaboration, Faculty of Medicine, Peradeniya, Sri Lanka. Heather M Wiseman BSc MSc Medical Toxicology Information Services, Guy’s and St Thomas’ NHS Foundation Trust, London SE1 9RT, UK. Contact details: Heather Wiseman Medical Toxicology Information Services Guy’s & St Thomas’ NHS Foundation Trust Mary Sheridan House Guy’s Hospital Great Maze Pond London SE1 9RT Tel 020 7188 7188 extn 51699 or 020 7188 0600 (admin office) Date 10th March 2010 succimer V 29 Nov 10.doc last saved: 29-Nov-10 11:30 Page 1 of 50 CONTENTS 1 Summary 2. Name of the focal point in WHO submitting or supporting the application 3. Name of the organization(s) consulted and/or supporting the application 4. International Nonproprietary Name (INN, generic name) of the medicine 5. Formulation proposed for inclusion 6. International availability 7. Whether listing is requested as an individual medicine or as an example of a therapeutic group 8. Public health relevance 8.1 Epidemiological information on burden of disease due to lead poisoning 8.2 Assessment of current use 8.2.1 Treatment of children with lead poisoning 8.2.2 Other indications 9. -

Sodium Cellulose Phosphate Sodium Edetate
Sevelamer/Sodium Edetate 1463 excreted by the kidneys. It may be used as a diagnostic is not less than 9.5% and not more than 13.0%, all calculated on supplement should not be given simultaneously with test for lead poisoning but measurement of blood-lead the dried basis. The calcium binding capacity, calculated on the sodium cellulose phosphate. dried basis, is not less than 1.8 mmol per g. concentrations is generally preferred. Sodium cellulose phosphate has also been used for the Sodium calcium edetate is also a chelator of other Adverse Effects and Precautions investigation of calcium absorption. heavy-metal polyvalent ions, including chromium. A Diarrhoea and other gastrointestinal disturbances have Preparations cream containing sodium calcium edetate 10% has been reported. USP 31: Cellulose Sodium Phosphate for Oral Suspension. been used in the treatment of chrome ulcers and skin Sodium cellulose phosphate should not be given to pa- Proprietary Preparations (details are given in Part 3) sensitivity reactions due to contact with heavy metals. tients with primary or secondary hyperparathyroidism, Spain: Anacalcit; USA: Calcibind. Sodium calcium edetate is also used as a pharmaceuti- hypomagnesaemia, hypocalcaemia, bone disease, or cal excipient and as a food additive. enteric hyperoxaluria. It should be used cautiously in pregnant women and children, since they have high Sodium Edetate In the treatment of lead poisoning, sodium calcium calcium requirements. Sodu edetynian. edetate may be given by intramuscular injection or by Patients should be monitored for electrolyte distur- intravenous infusion. The intramuscular route may be Эдетат Натрия bances. Uptake of sodium and phosphate may increase CAS — 17421-79-3 (monosodium edetate). -
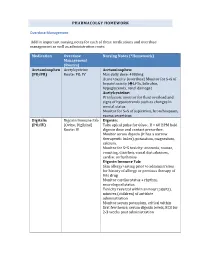
PHARMACOLGY HOMEWORK Overdose Management Add In
PHARMACOLGY HOMEWORK Overdose Management Add in important nursing notes for each of these medications and overdose management as well as administration route. Medication Overdose Nursing Notes (*Homework) Management (Routes) Acetaminophen Acetylcysteine Acetaminophen: (PO/PR) Route: PO, IV Max daily dose: 4000mg Acute toxicity (overdose) Monitor for S+S of hepatotoxicity (éLFTs, bilirubin, hypoglycemia, renal damage) Acetylcysteine: IV infusion: monitor for fluid overload and signs of hyponatremia such as changes in mental status Monitor for S+S of aspiration, bronchospasm, excess secretions Digitalis Digoxin Immune Fab Digoxin: (PO/IV) (Ovine, Digibind) Take apical pulse for 60sec. If < 60 BPM hold Route: IV digoxin dose and contact prescriber. Monitor serum digoxin (it has a narrow therapeutic index), potassium, magnesium, calcium. Monitor for S+S toxicity: anorexia, nausea, vomiting, diarrhea, visual disturbances, cardiac arrhythmias Digoxin Immune Fab: Skin allergy testing prior to administration for history of allergy or previous therapy of this drug Monitor cardiac status + rhythm, neurological status Toxicity reversal within an hour (adults), minutes (children) of antidote administration Monitor serum potassium, critical within first few hours; serum digoxin levels, ECG for 2-3 weeks post administration Heparin Protamine sulfate Heparin: (IV/SC) (IV) Monitor for spontaneous bleeding, thrombocytopenia Monitor aPTT levels Protamine Sulfate: Sudden drop in BP Monitor BP+ P q15-30 min Monitor aPTT Opioid Naloxone (IV-adults, Opioids: -

Product Information
PRODUCT INFORMATION DIGIFAB®1 POWDER FOR INJECTION (DIGOXIN-SPECIFIC ANTIBODY FRAGMENT F(Ab) (OVINE)) 1 NAME OF THE MEDICINE Digoxin-specific antibody fragment F(Ab) (Ovine) 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial of DIGIFAB Powder for Injection, will bind approximately 0.5 mg digoxin, contains 40 mg of digoxin- specific antibody fragment F(Ab). These fragments are obtained from blood of healthy sheep immunized with a digoxin derivative, digoxin- dicarboxymethoxylamine (DDMA), a digoxin analogue which contains the functionally essential cyclopentaperhydrophenanthrene:lactone ring moiety coupled to keyhole limpet hemocyanin (KLH). The final product is prepared by isolating the immunoglobulin fraction of the ovine serum, digesting it with papain and isolating the digoxin-specific Fab fragments by affinity chromatography. For the full list of excipients, see Section 6.1 List of Excipients. 3 PHARMACEUTICAL FORM DIGIFAB, digoxin-specific antibody fragment F(Ab) (Ovine) Powder for Injection, is a sterile, purified, lyophilized off-white powder of digoxin-specific antibody ovine Fab (monovalent) immunoglobulin fragments. The pH of the reconstituted solution is in between 4.5-5.5. The product is intended for intravenous administration after reconstitution with 4 mL of Sterile Water for Injection. 4 CLINICAL PARTICULARS 4.1 THERAPEUTIC INDICATIONS Digoxin-specific antibody fragment F(Ab) (Ovine) DIGIFAB is indicated for the treatment of known (or strongly suspected) life-threatening digoxin toxicity associated with ventricular arrhythmias, progressive bradycardia, or second or third degree heart block not responsive to atropine, and where additional measures besides withdrawal of digoxin and correction of serum electrolyte abnormalities are considered necessary. Consequences of multiple dosing with DIGIFAB have not been evaluated. -

Antidote List
Antidote List Recommended minimum amounts for hospital pharmacies to stock Call the Maryland Poison Center for expert advice on the treatment of all poisonings and overdoses: 1-800-222-1222 Poisoning Minimum Stocking Antidote Indication Recommendations Oral: 120 grams Acetylcysteine Acetaminophen IV: 96 grams Crotaline snake Antivenin, snake (CroFab®) 12 vials envenomation 1 vial Black widow spider (Note: Merck limits distribution Antivenin, black widow spider envenomation to cases of confirmed bites with symptoms only) Organophosphate & Atropine carbamate insecticides, nerve 165 mg gases Calcium chloride Calcium channel blockers 10 grams 2.25 grams Calcium disodium EDTA Lead (Available direct from ASD Specialty Healthcare) Hydrofluoric acid 1 kg (powder) Calcium gluconate Calcium channel blockers IV: 30 grams 1 kit Cyanide Antidote Kit Cyanide (or Hydroxocobalamin, see below) Cyproheptadine (Periactin®) Serotonin syndrome 80 mg Neuroleptic malignant Dantrolene syndrome, stimulant-induced 720 mg hyperthermia Deferoxamine mesylate Iron 8 grams (Desferal®) Digoxin Immune FAB Digoxin 20 vials (Digibind®, DigiFab®) Dimercaprol (BAL) Heavy metals 1.5 grams 1 | P a g e Maryland Poison Center Antidote List – continued Poisoning Minimum Stocking Antidote Indication Recommendations DMSA (Succimer, Chemet®) Heavy metals 2000 mg Folic acid Methanol IV: 150 mg Flumazenil (Romazicon®) Benzodiazepines 10 mg Fomepizole (Antizol®) Ethylene glycol, methanol 12 grams Beta blockers, Glucagon 50 mg calcium channel blockers Hydroxocobalamin (Cyanokit®) Cyanide -

Nova Scotia Antidote Program 2021 Quarterly Report #1 Jan 1, 2021 to Mar 31, 2021
Nova Scotia Antidote Program 2021 Quarterly Report #1 Jan 1, 2021 to Mar 31, 2021 The Nova Scotia Antidote Program is pleased to present another Quarterly Report, which provides information on changes and trends in antidote therapy and reports ongoing Provincial Antidote usage. Antidote usage Jan 1 to Mar 31, 2021 Western Northern Eastern Central IWK Quarterly Year to Date Zone Zone Zone Zone Total 6 7 7 20 2 42 42 Highlights of antidote use during the past 3 months A total of 42 antidotes were used in 32 different patient cases. Of these, 4 antidotes were used by community hospitals, 29 in regional facilities and 9 in tertiary hospitals. • Antidotes accessed in community hospitals: naloxone and sodium bicarbonate • Sodium Bicarbonate was used in six patients: Three patients with salicylate toxicity and three patients with evidence of sodium channel blockade (wide QRS). • Naloxone was reported as used for 18 patients with known or suspected opioid toxicity. o Six of these patients required a naloxone infusion, along with bolus dose(s). ISMP Alert: Methylene Blue The Institute for Safe Medication Practices Canada (ISMP) has issued a statement about “Hospital Readiness to Use the Antidote Methylene Blue”. There have been reported emergency situations where there was confusion as to the availability and dosing of methylene blue, resulting in delays in administration. (ISMP Canada Safety Bulletin; Vol. 21, Issue 5; May 6, 2021) Through our Provincial Antidote Program, emergency departments across Nova Scotia have sufficient stock (10 vials in Regional Kits, 3 vials in Community Kits) and access to online antidote monographs with information about methylene blue dosing and administration, adverse effects and monitoring parameters. -
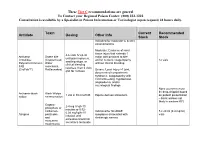
Antidote Stocking Tier C
These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Toxin Current Recommended Antidote Dosing Other Info Stock Stock Indicated for moderate to severe envenomations Moderate: Evidence of local tissue injury that extends 1 4-6 vials IV q2-4h Antivenin Snake bite major joint proximal to bite until pain improves, Crotalidae (Copperhead, and/or numeric coagulopathy 12 vials swelling stops, or Polyvalent Immune Water without clinical bleeding clinical bleeding FAB moccasins, resolves, then 2 vials (CroFab™) Rattlesnakes) Severe: Local injury >1 joint, q6h for 3 doses documented compartment syndrome, coagulopathy with clinical bleeding, hypotension, angioedema, and/or neurological findings None (currently must be drop-shipped based Antivenin-black Black Widow 1 vial in 50 ml of NS Equine derived antivenom on patient presentation widow envenomation – black widows not likely in western KY) Organo- 2-4 mg IV q5-10 phosphate or minutes or 0.02- carbamate Indicated for SLUDGE 5 x 20 ml (0.4 mg/ml) 0.08 mg/kg/hr IV Atropine pesticides symptoms associated with vials infusion until and cholinergic excess excessive bronchial muscarine secretions terminate mushrooms These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Antidote Current Recommended Toxin -

An Oral Treatment for Lead Toxicity Paul S
Postgrad Med J: first published as 10.1136/pgmj.67.783.63 on 1 January 1991. Downloaded from Postgrad MedJ (1991) 67, 63 - 65 D The Fellowship ofPostgraduate Medicine, 1991 Clinical Toxicology An oral treatment for lead toxicity Paul S. Thomas' and Charles Ashton2 'Northwick Park Hospital, Harrow, Middlesex HA] 3UJand2National Poisons Unit, Guy's Hospital, London SE], UK. Summary: Chronic lead poisoning has traditionally been treated by parenteral agents. We present a case where a comparison of ethylene diaminetetra-acetic acid was made with 2,3-dimethyl succinic acid (DMSA) which has the advantage oforal administration associated with little toxicity and appeared to be at least as efficacious. Introduction For many years the treatment of heavy metal ferase 112IU/I (normal<40). His electrolytes, poisoning has relied upon parenteral agents which urea, creatinine, clotting screening chest radio- themselves have a number of toxic side effects. We graph, computed tomographic head scan and lum- report a case ofplumbism which shows the benefits bar puncture were all normal. of using 2,3-dimethyl succinic acid (DMSA), a Re-evaluation revealed lead lines on his gingival treatment relatively new to Western countries. In margins and a urinary porphyrin screen was posi- by copyright. our case it was used in direct comparison to the tive indicating lead toxicity. Despite careful social, sodium-calcium salt of ethylene diaminetetra- occupational, and recreational history no cause for acetic acid, which has been the best available his excess lead intake could be found. As he was treatment. domiciled in India it was not possible to visit his home or workplace where he sold sarees. -
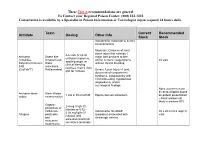
Antidote Stocking Tier A
These Tier A recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Toxin Current Recommended Antidote Dosing Other Info Stock Stock Indicated for moderate to severe envenomations Moderate: Evidence of local tissue injury that extends 1 4-6 vials IV q2-4h Antivenin Snake bite major joint proximal to bite until pain improves, Crotalidae (Copperhead, and/or numeric coagulopathy 24 vials swelling stops, or Polyvalent Immune Water without clinical bleeding clinical bleeding FAB moccasins, resolves, then 2 vials (CroFab™) Rattlesnakes) Severe: Local injury >1 joint, q6h for 3 doses documented compartment syndrome, coagulopathy with clinical bleeding, hypotension, angioedema, and/or neurological findings None (currently must be drop-shipped based Antivenin-black Black Widow 1 vial in 50 ml of NS Equine derived antivenom on patient presentation widow envenomation – black widows not likely in western KY) Organo- 2-4 mg IV q5-10 phosphate or minutes or 0.02- carbamate Indicated for SLUDGE 30 x 20 ml (0.4 mg/ml) 0.08 mg/kg/hr IV Atropine pesticides symptoms associated with vials infusion until and cholinergic excess excessive bronchial muscarine secretions terminate mushrooms These Tier A recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Antidote Current Recommended -

Antidotes Atropine Calcium
Version 2.9 Antidotes 4/10/2013 Atropine Indications AV conduction impairment: Cardiac glycosides , β-blockers , calcium channel blockers (CCBs) . Anticholinesterase inhibitors/Cholinergics: Organophosphates, carbamates Contraindications Relative: closed angle glaucoma, GIT obstruction, urinary obstruction Mechanism Competitive antagonist for ACh at muscarinic receptors. Pharmacokinetics Poor oral bioavailability, liver met, T ½=2-4hrs. Crosses BBB & placenta. 50% excreted unaltered. Administration AV conduction impairment: 0.6mg (20mcg/kg) IV repeated up to 3x Cholinergics: 1.2mg IV bolus, double dose q5mins until chest clear [also sBP>80mmHg, HR>80, dry axillae & no miosis]. Then infusion starting at ~10-20% of total loading dose (max 35mg/hr) Adverse Reactions Excessive dosage → Anticholinergic toxidrome. Calcium Indications CCB OD, HF exposure, hypocalcaemia, hyperkalaemia, iatrogenic hypermagnesaemia Contraindications Hypercalcaemia, ?digoxin toxicity - (contrary to traditional teaching, recently some evidence 2+ that Ca is not CI if on digoxin or even if digoxin toxic – however digoxin immune Fab & MgSO 4 10mmol might be preferred initially in the latter case) Mechanisms Restore low Ca2 + levels, binds F- ions, antagonises effects of high K + & Mg 2+ on heart. Administration Cardiac monitoring mandatory. CCBs: 20ml CaCl 2 IV (central line) or 60ml (1ml/kg) Ca gluconate IV (peripheral) over 5-10mins HF on skin: 2.5% Ca gel TOP OR local inj of 10% calcium gluconate (not fingers) OR Bier’s block with 2% Ca gluconate (i.e. 10ml of 10% in 40ml NS) for 20mins & release cuff OR same dose intra-arterially over 4 hrs & rpt prn. HF inhaled: Nebulised 2.5% Ca gluconate solution. HypoCa/HypoMg/HyperK: 5-10ml CaCl 2 or 10-20ml (1ml/kg) Ca gluconate IV over 5-10mins Adverse Reactions Transient hyperCa., vasodilatation, hypoBP, dysrhythmias, tissue damage from extravasated CaCl 2. -

Picture As Pdf Download
NOTABLE CASES Succimer therapy for congenital lead poisoning from maternal petrol sniffing An infant, born at 35 weeks’ gestation to a woman who sniffed petrol, had a cord blood lead level eight times the accepted limit. Treatment with oral dimercaptosuccinic acid promptly reduced his blood lead levels. To our knowledge, this is the first reported case of congenital lead poisoning secondary to maternal petrol sniffing. We suggest that at-risk pregnancies should be identified, cord blood lead levels tested, and chelation therapy and developmental follow-up offered to affected infants. (MJA 2006; 184: 84-85) Clinical record therapy is recommended for any child with blood lead levels of μ 3 A 27-year-old Indigenous woman, who had sniffed petrol since 2.16 mol/L and over. Although chelation therapy in early child- childhood, presented for antenatal care during her first pregnancy. hood lowers blood levels, recent studies have not shown signifi- At the age of 14 years, she had severe lead encephalopathy that led cant improvement in cognitive and behavioural measurements 3,4,7 to chronic neurological deficits, including permanent ataxia and compared with untreated children. memory impairment. Her serum lead levels at 8 and 35 weeks’ There are a few case reports of neonatal lead intoxication that gestation were raised at 1.48 and 2.21 μmol/L, respectively (recom- occurred from maternal exposure to lead through pica, home 8-10 mended level, р 0.48 μmol/L1). renovation, or use of contaminated herbal medications. Petrol At 35 weeks’ gestation, she went into spontaneous labour and sniffing is a form of substance misuse that is widespread in some gave birth vaginally to a boy. -

Oral Chelation Therapy for Patients with Lead Poisoning
ORAL CHELATION THERAPY FOR PATIENTS WITH LEAD POISONING Jennifer A. Lowry, MD Division of Clinical Pharmacology and Medical Toxicology The Children’s Mercy Hospitals and Clinics Kansas City, MO 64108 Tel: (816) 234-3059 Fax: (816) 855-1958 December 2010 1 TABLE OF CONTENTS 1. Background of Lead Poisoning …………………………………………………………...3 a. Clinical Significance of Lead Measurements …………………………………….3 b. Absorption of Lead and Its Internal Distribution Within the Body ………………3 c. Toxic Effects of Exposure to Lead in Children and Adults ………………………4 d. Reproductive and Developmental Effects………………………………………...5 e. Mechanisms of Lead Toxicity ……………………………………………………6 f. Concentration of Lead in Blood Deemed Safe for Children/Adults………………6 g. Use of Blood Lead Measurements as a Marker of Lead Exposure ……………….7 2. Management of the Child with Elevated Blood Lead Concentrations …………………...8 a. Decreasing Exposure……………………………………………………………...8 b. Chelation Therapy…………………………………………………………………8 3. Oral Chelation Therapy …………………………………………………………………...8 a. Meso-2,3 dimercaptosuccinic acid (DMSA, Succimer) …………………………8 i. Pharmacokinetics………………………………………………………….9 ii. Dosing …………………………………………………………………….9 iii. Efficacy……………………………………………………………………9 iv. Safety…………………………………………………………………….11 b. Racemic-2,3-dimercapto-1-propanesulfonic acid (DMPS, Unithiol)……………11 i. Pharmacokinetics………………………………………………………...12 ii. Dosing ……………………………………………………………………12 iii. Efficacy…………………………………………………………………..12 iv. Safety…………………………………………………………………….12 c. Penicillamine……………………………………………………………………..12 i. Pharmacokinetics………………………………………………………...13