Digifab Digoxin Immune Fab (Ovine)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uses and Administration Adverse Effects and Precautions Pharmacokinetics Uses and Administration
1549 The adverse effects of dicobalt edetate are more severe in year-old child: case report and review of literature. Z Kardiol 2005; 94: References. 817-2 3. the absence of cyanide. Therefore, dicobalt edetate should I. Schaumann W, et a!. Kinetics of the Fab fragments of digoxin antibodies and of bound digoxin in patients with severe digoxin intoxication. Eur 1 not be given unless cyanide poisoning is confirmed and 30: Administration in renal impairment. In renal impairment, Clin Pharmacol 1986; 527-33. poisoning is severe such as when consciousness is impaired. 2. Ujhelyi MR, Robert S. Pharmacokinetic aspects of digoxin-specific Fab elimination of antibody-bound digoxin or digitoxin is therapy in the management of digitalis toxicity. Clin Pharmacokinet 1995; Oedema. A patient with cyanide toxicity developed delayed1·3 and antibody fragments can be detected in the 28: 483-93. plasma for 2 to 3 weeks after treatment.1 The rebound in 3. Renard C, et al. Pharmacokinetics of dig,'><i<1-sp,eei1ie Fab: effects of severe facial and pulmonary oedema after treatment with decreased renal function and age. 1997; 44: 135-8. dicobalt edetate.1 It has been suggested that when dicobalt free-digoxin concentrations that has been reported after edetate is used, facilities for intubation and resuscitation treatment with digoxin-specific antibody fragments (see P (Jrations should be immediately available. Poisoning, below), occurred much later in patients with r.�p renal impairment than in those with normal renal func Proprietary Preparations (details are given in Volume B) 1. Dodds C, McKnight C. Cyanide toxicity after immersion and the hazards tion. -
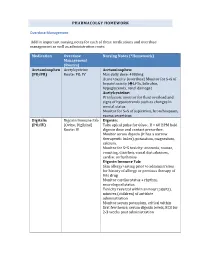
PHARMACOLGY HOMEWORK Overdose Management Add In
PHARMACOLGY HOMEWORK Overdose Management Add in important nursing notes for each of these medications and overdose management as well as administration route. Medication Overdose Nursing Notes (*Homework) Management (Routes) Acetaminophen Acetylcysteine Acetaminophen: (PO/PR) Route: PO, IV Max daily dose: 4000mg Acute toxicity (overdose) Monitor for S+S of hepatotoxicity (éLFTs, bilirubin, hypoglycemia, renal damage) Acetylcysteine: IV infusion: monitor for fluid overload and signs of hyponatremia such as changes in mental status Monitor for S+S of aspiration, bronchospasm, excess secretions Digitalis Digoxin Immune Fab Digoxin: (PO/IV) (Ovine, Digibind) Take apical pulse for 60sec. If < 60 BPM hold Route: IV digoxin dose and contact prescriber. Monitor serum digoxin (it has a narrow therapeutic index), potassium, magnesium, calcium. Monitor for S+S toxicity: anorexia, nausea, vomiting, diarrhea, visual disturbances, cardiac arrhythmias Digoxin Immune Fab: Skin allergy testing prior to administration for history of allergy or previous therapy of this drug Monitor cardiac status + rhythm, neurological status Toxicity reversal within an hour (adults), minutes (children) of antidote administration Monitor serum potassium, critical within first few hours; serum digoxin levels, ECG for 2-3 weeks post administration Heparin Protamine sulfate Heparin: (IV/SC) (IV) Monitor for spontaneous bleeding, thrombocytopenia Monitor aPTT levels Protamine Sulfate: Sudden drop in BP Monitor BP+ P q15-30 min Monitor aPTT Opioid Naloxone (IV-adults, Opioids: -

Product Information
PRODUCT INFORMATION DIGIFAB®1 POWDER FOR INJECTION (DIGOXIN-SPECIFIC ANTIBODY FRAGMENT F(Ab) (OVINE)) 1 NAME OF THE MEDICINE Digoxin-specific antibody fragment F(Ab) (Ovine) 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial of DIGIFAB Powder for Injection, will bind approximately 0.5 mg digoxin, contains 40 mg of digoxin- specific antibody fragment F(Ab). These fragments are obtained from blood of healthy sheep immunized with a digoxin derivative, digoxin- dicarboxymethoxylamine (DDMA), a digoxin analogue which contains the functionally essential cyclopentaperhydrophenanthrene:lactone ring moiety coupled to keyhole limpet hemocyanin (KLH). The final product is prepared by isolating the immunoglobulin fraction of the ovine serum, digesting it with papain and isolating the digoxin-specific Fab fragments by affinity chromatography. For the full list of excipients, see Section 6.1 List of Excipients. 3 PHARMACEUTICAL FORM DIGIFAB, digoxin-specific antibody fragment F(Ab) (Ovine) Powder for Injection, is a sterile, purified, lyophilized off-white powder of digoxin-specific antibody ovine Fab (monovalent) immunoglobulin fragments. The pH of the reconstituted solution is in between 4.5-5.5. The product is intended for intravenous administration after reconstitution with 4 mL of Sterile Water for Injection. 4 CLINICAL PARTICULARS 4.1 THERAPEUTIC INDICATIONS Digoxin-specific antibody fragment F(Ab) (Ovine) DIGIFAB is indicated for the treatment of known (or strongly suspected) life-threatening digoxin toxicity associated with ventricular arrhythmias, progressive bradycardia, or second or third degree heart block not responsive to atropine, and where additional measures besides withdrawal of digoxin and correction of serum electrolyte abnormalities are considered necessary. Consequences of multiple dosing with DIGIFAB have not been evaluated. -

Antidote List
Antidote List Recommended minimum amounts for hospital pharmacies to stock Call the Maryland Poison Center for expert advice on the treatment of all poisonings and overdoses: 1-800-222-1222 Poisoning Minimum Stocking Antidote Indication Recommendations Oral: 120 grams Acetylcysteine Acetaminophen IV: 96 grams Crotaline snake Antivenin, snake (CroFab®) 12 vials envenomation 1 vial Black widow spider (Note: Merck limits distribution Antivenin, black widow spider envenomation to cases of confirmed bites with symptoms only) Organophosphate & Atropine carbamate insecticides, nerve 165 mg gases Calcium chloride Calcium channel blockers 10 grams 2.25 grams Calcium disodium EDTA Lead (Available direct from ASD Specialty Healthcare) Hydrofluoric acid 1 kg (powder) Calcium gluconate Calcium channel blockers IV: 30 grams 1 kit Cyanide Antidote Kit Cyanide (or Hydroxocobalamin, see below) Cyproheptadine (Periactin®) Serotonin syndrome 80 mg Neuroleptic malignant Dantrolene syndrome, stimulant-induced 720 mg hyperthermia Deferoxamine mesylate Iron 8 grams (Desferal®) Digoxin Immune FAB Digoxin 20 vials (Digibind®, DigiFab®) Dimercaprol (BAL) Heavy metals 1.5 grams 1 | P a g e Maryland Poison Center Antidote List – continued Poisoning Minimum Stocking Antidote Indication Recommendations DMSA (Succimer, Chemet®) Heavy metals 2000 mg Folic acid Methanol IV: 150 mg Flumazenil (Romazicon®) Benzodiazepines 10 mg Fomepizole (Antizol®) Ethylene glycol, methanol 12 grams Beta blockers, Glucagon 50 mg calcium channel blockers Hydroxocobalamin (Cyanokit®) Cyanide -

Nova Scotia Antidote Program 2021 Quarterly Report #1 Jan 1, 2021 to Mar 31, 2021
Nova Scotia Antidote Program 2021 Quarterly Report #1 Jan 1, 2021 to Mar 31, 2021 The Nova Scotia Antidote Program is pleased to present another Quarterly Report, which provides information on changes and trends in antidote therapy and reports ongoing Provincial Antidote usage. Antidote usage Jan 1 to Mar 31, 2021 Western Northern Eastern Central IWK Quarterly Year to Date Zone Zone Zone Zone Total 6 7 7 20 2 42 42 Highlights of antidote use during the past 3 months A total of 42 antidotes were used in 32 different patient cases. Of these, 4 antidotes were used by community hospitals, 29 in regional facilities and 9 in tertiary hospitals. • Antidotes accessed in community hospitals: naloxone and sodium bicarbonate • Sodium Bicarbonate was used in six patients: Three patients with salicylate toxicity and three patients with evidence of sodium channel blockade (wide QRS). • Naloxone was reported as used for 18 patients with known or suspected opioid toxicity. o Six of these patients required a naloxone infusion, along with bolus dose(s). ISMP Alert: Methylene Blue The Institute for Safe Medication Practices Canada (ISMP) has issued a statement about “Hospital Readiness to Use the Antidote Methylene Blue”. There have been reported emergency situations where there was confusion as to the availability and dosing of methylene blue, resulting in delays in administration. (ISMP Canada Safety Bulletin; Vol. 21, Issue 5; May 6, 2021) Through our Provincial Antidote Program, emergency departments across Nova Scotia have sufficient stock (10 vials in Regional Kits, 3 vials in Community Kits) and access to online antidote monographs with information about methylene blue dosing and administration, adverse effects and monitoring parameters. -
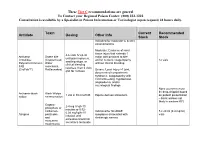
Antidote Stocking Tier C
These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Toxin Current Recommended Antidote Dosing Other Info Stock Stock Indicated for moderate to severe envenomations Moderate: Evidence of local tissue injury that extends 1 4-6 vials IV q2-4h Antivenin Snake bite major joint proximal to bite until pain improves, Crotalidae (Copperhead, and/or numeric coagulopathy 12 vials swelling stops, or Polyvalent Immune Water without clinical bleeding clinical bleeding FAB moccasins, resolves, then 2 vials (CroFab™) Rattlesnakes) Severe: Local injury >1 joint, q6h for 3 doses documented compartment syndrome, coagulopathy with clinical bleeding, hypotension, angioedema, and/or neurological findings None (currently must be drop-shipped based Antivenin-black Black Widow 1 vial in 50 ml of NS Equine derived antivenom on patient presentation widow envenomation – black widows not likely in western KY) Organo- 2-4 mg IV q5-10 phosphate or minutes or 0.02- carbamate Indicated for SLUDGE 5 x 20 ml (0.4 mg/ml) 0.08 mg/kg/hr IV Atropine pesticides symptoms associated with vials infusion until and cholinergic excess excessive bronchial muscarine secretions terminate mushrooms These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Antidote Current Recommended Toxin -
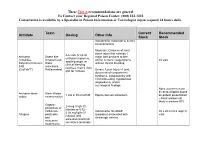
Antidote Stocking Tier A
These Tier A recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Toxin Current Recommended Antidote Dosing Other Info Stock Stock Indicated for moderate to severe envenomations Moderate: Evidence of local tissue injury that extends 1 4-6 vials IV q2-4h Antivenin Snake bite major joint proximal to bite until pain improves, Crotalidae (Copperhead, and/or numeric coagulopathy 24 vials swelling stops, or Polyvalent Immune Water without clinical bleeding clinical bleeding FAB moccasins, resolves, then 2 vials (CroFab™) Rattlesnakes) Severe: Local injury >1 joint, q6h for 3 doses documented compartment syndrome, coagulopathy with clinical bleeding, hypotension, angioedema, and/or neurological findings None (currently must be drop-shipped based Antivenin-black Black Widow 1 vial in 50 ml of NS Equine derived antivenom on patient presentation widow envenomation – black widows not likely in western KY) Organo- 2-4 mg IV q5-10 phosphate or minutes or 0.02- carbamate Indicated for SLUDGE 30 x 20 ml (0.4 mg/ml) 0.08 mg/kg/hr IV Atropine pesticides symptoms associated with vials infusion until and cholinergic excess excessive bronchial muscarine secretions terminate mushrooms These Tier A recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Antidote Current Recommended -

Antidotes Atropine Calcium
Version 2.9 Antidotes 4/10/2013 Atropine Indications AV conduction impairment: Cardiac glycosides , β-blockers , calcium channel blockers (CCBs) . Anticholinesterase inhibitors/Cholinergics: Organophosphates, carbamates Contraindications Relative: closed angle glaucoma, GIT obstruction, urinary obstruction Mechanism Competitive antagonist for ACh at muscarinic receptors. Pharmacokinetics Poor oral bioavailability, liver met, T ½=2-4hrs. Crosses BBB & placenta. 50% excreted unaltered. Administration AV conduction impairment: 0.6mg (20mcg/kg) IV repeated up to 3x Cholinergics: 1.2mg IV bolus, double dose q5mins until chest clear [also sBP>80mmHg, HR>80, dry axillae & no miosis]. Then infusion starting at ~10-20% of total loading dose (max 35mg/hr) Adverse Reactions Excessive dosage → Anticholinergic toxidrome. Calcium Indications CCB OD, HF exposure, hypocalcaemia, hyperkalaemia, iatrogenic hypermagnesaemia Contraindications Hypercalcaemia, ?digoxin toxicity - (contrary to traditional teaching, recently some evidence 2+ that Ca is not CI if on digoxin or even if digoxin toxic – however digoxin immune Fab & MgSO 4 10mmol might be preferred initially in the latter case) Mechanisms Restore low Ca2 + levels, binds F- ions, antagonises effects of high K + & Mg 2+ on heart. Administration Cardiac monitoring mandatory. CCBs: 20ml CaCl 2 IV (central line) or 60ml (1ml/kg) Ca gluconate IV (peripheral) over 5-10mins HF on skin: 2.5% Ca gel TOP OR local inj of 10% calcium gluconate (not fingers) OR Bier’s block with 2% Ca gluconate (i.e. 10ml of 10% in 40ml NS) for 20mins & release cuff OR same dose intra-arterially over 4 hrs & rpt prn. HF inhaled: Nebulised 2.5% Ca gluconate solution. HypoCa/HypoMg/HyperK: 5-10ml CaCl 2 or 10-20ml (1ml/kg) Ca gluconate IV over 5-10mins Adverse Reactions Transient hyperCa., vasodilatation, hypoBP, dysrhythmias, tissue damage from extravasated CaCl 2. -

ANTIDOTE CHART ***This Poster Is Not Intended for Individual Patient Care
ANTIDOTE CHART ***This poster is not intended for individual patient care. If you are caring for a known or suspected poisoned patient, contact your regional poison center for patient-specific recommendations*** Stocking Recommendations Antidote Indication (100 kg patient) Notes 8 hours 24 hours Acetylcysteine (intravenous) 22 grams 30 grams Acetaminophen toxicity Administer intravenously (IV) for hepatic failure Acetylcysteine (oral) 28 grams 56 grams Production discontinued by manufacturer; some limited Antivenin (Latrodectus mactans) Black widow spider envenomation 1 vial 1 vial supply remains Organophosphate/carbamate pesticide and nerve Atropine sulfate 45 mg 165 mg agent toxicity Calcium chloridea,b 10 grams 10 grams Administer by central venous route if possible Fluoride, calcium channel blocker toxicity Calcium gluconatea,b 30 grams 30 grams May be given by IV, subcutaneous routes Calcium disodium EDTA Lead toxicity 0.75 grams 2.25 grams Calcium trisodium pentetate Internal contamination with plutonium, americium, 1 gram 1 gram (calcium DTPA) or curium Crotalidae Polyvalent Immune North American crotaline snake envenomation 12 vials 18 vials Fab (ovine) (CroFab®) Cyproheptadineb Serotonin toxicity 20 mg 36 mg Dantroleneb Malignant hyperthermia 800 mg 2,000 mg Make available where general anesthesia is performed Deferoxamine mesylate Iron toxicity 12 grams 36 grams D50 as initial treatment, may follow with lower Dextrose (D50) Hypoglycemia 250 grams 250 grams concentrations Digoxin immune Fab Cardiac glycoside toxicity 15 vials -

Antidote Shortages- Impact and Response BOD APPROVED 11.15.12
POSITION STATEMENT Antidote Shortages: Impact and Response Disclaimer While individual practitioners may differ, these are the positions of the ACMT and the AACT at the time written, after a review of the issue and pertinent literature. Background The recently passed 2012 Food and Drug Administration Safety and Innovation Act1 requires manufacturers to notify the Secretary of Health and Human Services of any significant disruption in the availability of emergency, life-supporting medications. The Act also requires the Secretary to establish a task force to develop and implement a strategic plan for enhancing the response to preventing and mitigating drug shortages and requires the Comptroller General of the United States to conduct a study to examine the cause of drug shortages and formulate recommendations on how to prevent or alleviate such shortages. Inadequate antidote and antivenom supply to treat poisonings and envenomations has long been recognized by the toxicology and emergency medicine communities.2-8 A recent worsening trend of antidotes and antivenoms in critically short or absent supply places patients at increased risk for adverse outcomes. Shortages have included, but have not been limited to, atropine, benzodiazepines (diazepam, midazolam, et al.), black widow spider (Latrodectus mactans) antivenin, CaNa2EDTA, dexrazoxane, digoxin immune Fab, epinephrine, ethanol, etomidate, intravenous fat emulsion (20%), leucovorin, methylene blue, N-acetylcysteine, naloxone, North American coral snake (Micrurus fulvius) antivenin, octreotide, sodium bicarbonate, and vitamin K.9,10 Significant economic disincentives exist to produce rarely used drugs (such as antidotes). While the FDA’s Orphan Drug program provides some inducement, many antidotes and antivenoms are provided by a single entity. -

Iowa Poison Control Center
Iowa Poison Control Center Recommended Antidote Stocking for Hospitals with an Emergency Department *** Contact the IPCC for treatment recommendations at 800-222-1222 *** These are suggested antidote stocking levels for managing one adult patient for either 8 or 24 hours. Depending upon the severity of the exposure, the actual amount of antidote required for a specific case may be greater than what is listed below. Each hospital needs to decide its own appropriate antidote stocking levels based on the hospital’s: location, history of antidote use, patient population, patient census, industry in the area, pharmacy budget, and availability of antidotes from local resources (e.g. hospital sharing). Antidote Poisoning Suggested Minimum Access Comments Indication Stocking Level Priority Activated charcoal GI decontamination 100 gm Immediately (aqueous) N-Acetylcysteine IV Acetaminophen, 24 hours: 30 gm or Within 1 formulation Other hepatotoxins 5x30mL (20%) vials hour (AcetadoteTM) N-Acetylcysteine Acetaminophen, 8 hours: 30 gm or Within 1 Oral formulation Other hepatotoxins 5x30mL (20%) vials hour (Mucomyst®, 24 hours: 60 gm or Cetylev®) 10x30mL (20%) vials Antivenin, Crotalidae Pit Viper 8 hours: 12 vials Within 1 Polycalent Immune envenomation (e.g. 24 hours: 18 vials hour Fab – Ovine rattlesnakes, (CroFab®) cottonmouths, & copperheads) Antivenin Black Widow Spider 8 hours: 1 vial Special access – (Lactrodectus envenomation 24 hours: 1 vial contact manufacturer mactans)®, Black Merck at (800)-672- Widow Spider 6372 Atropine sulfate Organophosphate & 8 hours: 50 mg Immediately carbamate 24 hours: 175 mg insecticides Benztropine Acute dystonia 6 mg (3x2 ml, 1 mg/ml) (Cogentin®) Botulinum antitoxin; Botulism Available only from pentavalent ABCDE CDC. Contact county or state Department of Public Health. -

Availability of Antidotes at Acute Care Hospitals in Ontario
Availability of antidotes at acute care hospitals in Ontario Research Recherche David N. Juurlink,*‡¶** Michael A. McGuigan,†¶ Thomas W. Paton,§ Donald A. Redelmeier*‡** From the Departments of Abstract *Medicine and †Pediatrics, University of Toronto; Background: Acutely poisoned patients sometimes require immediate treatment ‡the Clinical Epidemiology with an antidote, and delays in treatment can be fatal. We sought to determine and Health Care Research the availability of 10 antidotes at acute care hospitals in Ontario. Program and §Department Methods: Mailed questionnaire with repeated reminders to pharmacy directors at of Pharmacy, Sunnybrook & all acute care hospitals in Ontario. Women’s College Health Results: Responses were obtained from 179 (97%) of 184 hospitals. Only 9% of Sciences Centre; ¶the Ontario the hospitals stocked an adequate supply of digoxin immune Fab antibody frag- Regional Poison Centre; and ments, a life-saving antidote for patients with severe digoxin toxicity, whereas **the Institute for Clinical most of the hospitals stocked sufficient supplies of ipecac syrup (88%) and Evaluative Sciences, Toronto, flumazenil (92%), arguably the least crucial antidotes in the survey. Only 1 hos- Ont. At the time of writing, pital stocked adequate amounts of all 10 antidotes. Certain hospital characteris- Dr. McGuigan was Medical tics were associated with adequate antidote stocking (increased annual emer- Director of the Ontario gency department volume, teaching hospital status and designation as a trauma Regional Poison Centre; he centre). Conversely, antidote supplies were particularly deficient at small hospi- is now Medical Director of tals and, paradoxically, geographically isolated facilities (those most reliant on the Long Island Regional their own inventory). The cost of antidotes correlated only weakly with stocking Poison Control Center, rates, and many examples of excessive antidote stocking were identified.