Antidote Stocking Tier A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cyanide Poisoning and How to Treat It Using CYANOKIT (Hydroxocobalamin for Injection) 5G
Cyanide Poisoning and How to Treat It Using CYANOKIT (hydroxocobalamin for injection) 5g 1. CYANOKIT (single 5-g vial) [package insert]. Columbia, MD: Meridian Medical Technologies, Inc.; 2011. Please see Important Safety Information on slides 3-4 and full Prescribing Information for CYANOKIT starting on slide 33. CYANOKIT is a registered trademark of SERB Sarl, licensed by Meridian Medical Technologies, Inc., a Pfizer company. Copyright © 2015 Meridian Medical Technologies, Inc., a Pfizer company. All rights reserved. CYK783109-01 November/2015. Indication and Important Safety Information……………………………………………………………………………….………..…..3 . Identifying Cyanide Poisoning……………………………………………………………………………………………………………….…………….….5 . How CYANOKIT (hydroxocobalamin for injection) Works……………………………………………………………….12 . The Specifics of CYANOKIT…………………………………………………………………………………………………………………………….………17 . Administering CYANOKIT………………………………………………………………………………………………………………………………..……….21 . Storage and Disposal of CYANOKIT…................................................................................................................................26 . Grant Information for CYANOKIT……………………………………………………………………………………………………………………....30 . Full Prescribing Information………………………………………………………………………………………………….………………………………33 Please see Important Safety Information on slides 3-4 and full Prescribing Information for CYANOKIT starting on slide 33. CYANOKIT (hydroxocobalamin for injection) 5 g for intravenous infusion is indicated for the treatment of known or suspected cyanide poisoning. -

Evaluation of Neuropharmacological Activity of Hydroalcoholic Extract of Fruits of Trapa Bispinosa in Laboratory Animals
International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 2, Suppl 2, 2010 Research Article EVALUATION OF NEUROPHARMACOLOGICAL ACTIVITY OF HYDROALCOHOLIC EXTRACT OF FRUITS OF TRAPA BISPINOSA IN LABORATORY ANIMALS VYAWAHARE N. S.a AND AMBIKAR D. B.* b aDepartment of Pharmacology, AISSMS College of Pharmacy, Kennedy Road, Pune 411 001, India. bDepartment of Pharmacology, Marathwada Mitra Mandals College of Pharmacy, Thergaon, Pune 411033, India. Email: [email protected] Received: 26 Dec 2009, Revised and Accepted:19 Jan 2010 ABSTRACT The present investigation deals with evaluation of neuropharmacological activity of different doses (100, 250, 500mg/kg, p.o.) of hydroalcoholic extract of Trapa bispinosa (TB) in laboratory animals. The effects of extract on various parameters like motor coordination, spontaneous locomotor activity, object recognition, transfer latency, anxiolytic activity, analgesic activity, sodium nitrite induced respiratory arrest and hypoxic stress etc was studied. The TB (250 & 500mg/kg) found to decrease time required to occupy the central platform (transfer latency) in the elevated plus maze and to increase discrimination index in the object recognition test, indicating nootropic activity. TB (250 and 500mg/kg) showed significant increase in reaction time in hot plate analgesic activity. Moreover it also showed significant reduction in spontaneous locomotor activity and latency to death in sodium nitrite induced respiratory arrest. To conclude hydroalcoholic extract of TB possesses significant facilitation of learning and memory which may be due to enhanced cholinergic function. TB also showed significant analgesic activity. Key words: Trapa bispinosa, Nootropic, Analgesic. Chemicals and drugs INTRODUCTION Sodium nitrate was purchased from Loba chemicals, Mumbai. Medicinal herbs constitute the cornerstone of traditional medicinal Diazepam injection, pentazocin injection, piracetam syrup, was practice worldwide. -

Letter to the Editor Effect of N-Acetylcysteine And
Indian J Physiol Pharmacol 1999; 43 (4): 518-520 LETTER TO THE EDITOR EFFECT OF N-ACETYLCYSTEINE AND SODIUM NITRITE ON MYOCARDIAL REPERFUSION INJURY Sir, (Received on May 22, 1999) Results of experimental studies suggest India). Heart was suspended in pericardial the involvement of oxygen free radicals cradle after left sided thoracotomy at fourth (OFR) in myocardial reperfusion injury 0, intercostal space. A polythene catheter 2). Ischemia is known to deplete endogenous (1.5 mm inner diameter) was placed in the antioxidants like superoxide dismutase, left ventricle through apex t~ [ecord glutathione, catalase 0, 2) and makes ventricular pressure changes on Grass 16 myocardial, cell vulnerable to oxygen free channel polygraph (Model 79D, USA) by radical (OFR) induced damage 0, 2). OFR using Gould pressure transducer. Heart rate can damage endothelial cells at the time of and ST segment changes were monitored in reperfusion (3). Endothelial cells synthesise standard limb leads on BPL (India) and form nitric oxide which is endothelium, electrocardiograph. Another two catheters derived relaxation factor (4). Acidified were placed in the left atrium for infusion sodium nitrite (Na Oz) at pH-2 forms nitric of saline, drug or acidified N aNOz' Left oxide (5) which can inactivate superoxide anterior descending (LAD) coronary artery radicals (5, 6). N-acetylcysteine (NAC) is was dissected free above first diagonal known to replenish glutathione stores, branch and below the origin of left increase superoxide dismutase activity, circumflex artery. Duration of LAD coronary scanvenge hydroxyl radicals and interfere artery occlusion was 90-min. Following with autocatalytic lipid peroxidation (7, 8). completion of occlusion, reperfusion of the In our previous studies we have shown the myocardium was initiated by removing the individual effects of NAC and NaNOz on vessel occludeI'. -

Long-Term N-Acetylcysteine and L-Arginine Administration Reduces Endothelial Activation and Systolic Blood Pressure in Hypertensive Patients with Type 2 Diabetes
Emerging Treatments and Technologies ORIGINAL ARTICLE Long-Term N-Acetylcysteine and L-Arginine Administration Reduces Endothelial Activation and Systolic Blood Pressure in Hypertensive Patients With Type 2 Diabetes 1 1 VALENTINO MARTINA, MD PAOLA MASSARENTI, MD ardiovascular complications repre- 1 1 ANDI MASHA, MD FABIO SETTANNI, PHD sent 80% of the causes of death in 1 2 VALENTINA RAMELLA GIGLIARDI, MD LARA DELLA CASA, PHD 1 patients with type 2 diabetes. Several OREDANA ROCATO MD 2 C L B , STEFANIA BERGAMINI, PHD causes may explain this mortality excess. 3 2 ENZO MANZATO, MD, PHD NNA ANNONE MD, PHD 1 A I , Among these, the decreased availability of ARRIGO BERCHIO, MD nitric oxide (NO) has increasingly gained credit. In fact, reduced NO availability has been demonstrated not only in diabe- OBJECTIVE — Reactive oxygen and nitric oxide (NO) have recently been considered to be tes (1) but also in other diseases, such as involved in the cardiovascular complications of patients with type 2 diabetes, as NO is thought atherosclerosis and hypertension, known to lose its beneficial physiological effects in the presence of oxygen radicals. For this reason, we to be associated with increased mortality tested the effects of L-arginine (ARG) and N-acetylcysteine (NAC) administration in increasing due to cardiovascular causes (2,3). NO is NO bioavailability by reducing free radical formation. crucial for regulating the vascular tone RESEARCH DESIGN AND METHODS — A double-blind study was performed on 24 and maintaining the intrinsic thrombore- male patients with type 2 diabetes and hypertension divided into two groups of 12 patients that sistant and atheroprotective properties of randomly received either an oral supplementation of placebo or NAC ϩ ARG for 6 months. -

Uses and Administration Adverse Effects and Precautions Pharmacokinetics Uses and Administration
1549 The adverse effects of dicobalt edetate are more severe in year-old child: case report and review of literature. Z Kardiol 2005; 94: References. 817-2 3. the absence of cyanide. Therefore, dicobalt edetate should I. Schaumann W, et a!. Kinetics of the Fab fragments of digoxin antibodies and of bound digoxin in patients with severe digoxin intoxication. Eur 1 not be given unless cyanide poisoning is confirmed and 30: Administration in renal impairment. In renal impairment, Clin Pharmacol 1986; 527-33. poisoning is severe such as when consciousness is impaired. 2. Ujhelyi MR, Robert S. Pharmacokinetic aspects of digoxin-specific Fab elimination of antibody-bound digoxin or digitoxin is therapy in the management of digitalis toxicity. Clin Pharmacokinet 1995; Oedema. A patient with cyanide toxicity developed delayed1·3 and antibody fragments can be detected in the 28: 483-93. plasma for 2 to 3 weeks after treatment.1 The rebound in 3. Renard C, et al. Pharmacokinetics of dig,'><i<1-sp,eei1ie Fab: effects of severe facial and pulmonary oedema after treatment with decreased renal function and age. 1997; 44: 135-8. dicobalt edetate.1 It has been suggested that when dicobalt free-digoxin concentrations that has been reported after edetate is used, facilities for intubation and resuscitation treatment with digoxin-specific antibody fragments (see P (Jrations should be immediately available. Poisoning, below), occurred much later in patients with r.�p renal impairment than in those with normal renal func Proprietary Preparations (details are given in Volume B) 1. Dodds C, McKnight C. Cyanide toxicity after immersion and the hazards tion. -

Sodium Nitrite and Sodium Thiosulfate
PATIENT & CAREGIVER EDUCATION Sodium Nitrite and Sodium Thiosulfate This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Nithiodote Warning This drug may cause low blood pressure and a red blood cell problem called methemoglobinemia. These may be life-threatening. This drug is only for use when cyanide poisoning is life-threatening. This drug must be used with care if it is not known if cyanide poisoning has happened. Talk with the doctor. Tell the doctor if your child has inhaled a lot of smoke or if your child has any of these health problems: Anemia, heart problems, lack of a certain enzyme called congenital methemoglobin reductase deficiency, or lung problems. What is this drug used for? It is used to treat cyanide poisoning. What do I need to tell the doctor BEFORE my child takes this drug? If your child is allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell the doctor about the allergy and what signs your child had. Sodium Nitrite and Sodium Thiosulfate 1/6 If your child is breast-feeding a baby: Be sure your child does not breast-feed a baby while taking this drug. This drug may interact with other drugs or health problems. Tell the doctor and pharmacist about all of your child’s drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe to give this drug with all of your child’s other drugs and health problems. -

Annex 1: Standard Operation Procedures (SOP) for Environmental Monitoring
Project Completion Report Annex Annex 1: Standard Operation Procedures (SOP) For Environmental Monitoring 1.5 Air Quality Nippon Koei Co., Ltd. The Capacity Development of Environmental Monitoring at Directorates Standard Operation Procedure (SOP)-01 Sampling and Concentration Calculation Procedure of Nitrogen Oxide (NOX) Air Quality Analysis Date of preparing SOP Work Sheet (Air quality) Measuring substance Nitrogen Oxide (NOX) Measurement method Saltzman Reaction Method 1- Measurement flowchart and main items (1) Preparation of equipments and tools (Reference) ① Selection of absorption tube. ② Preparation of the handy sampler. (2) Adjustment of the reagents ① Making the solution of 0.1% N-(1-Naphthyl) ethylenediamine dihydrochloride (hereafter, it is called NEDA solution) ② Making the absorption solution ③ Making the standard undiluted solution NaNO2 (2.03 g/L) ④ Making the standard solution NaNO2 (0.0203 g/L) ⑤ Making oxidation solution (3) Carrying out the measurement ① Establishment of the sampling locations ② Collecting the samples (sampling) ③ Making sampling solution for the analysis purpose (4) Making the analysis ① Measuring the absorbance of the collected samples ② Measuring the absorbance of the standard solution NaNO2 (5) Calculating the concentration ① Drawing the calibration curve ② Calculating the concentration of NO2 ③ Calculating the concentration of NO - 1 - 2.Measurement principle If we put the air sample which contains NO2 in an absorption coloring solution (N-(1-Naphthyl) ethylenediamine dihydrochloride, Sulfanilic acid, and Acetic acid mixture solution), we will get an orange-red azo-dye (dyestuff) that is proportional to NO2 amount. By measuring the absorbance of the coloring solution we can calculate NO2 concentration. Because NO does not react with the absorption coloring solution, it is oxidized to NO2 by using potassium permanganate (after adding sulfuric acid), then we can measure it by the same method mentioned-above. -
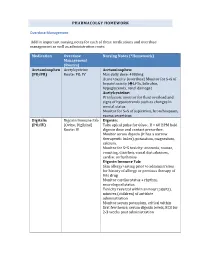
PHARMACOLGY HOMEWORK Overdose Management Add In
PHARMACOLGY HOMEWORK Overdose Management Add in important nursing notes for each of these medications and overdose management as well as administration route. Medication Overdose Nursing Notes (*Homework) Management (Routes) Acetaminophen Acetylcysteine Acetaminophen: (PO/PR) Route: PO, IV Max daily dose: 4000mg Acute toxicity (overdose) Monitor for S+S of hepatotoxicity (éLFTs, bilirubin, hypoglycemia, renal damage) Acetylcysteine: IV infusion: monitor for fluid overload and signs of hyponatremia such as changes in mental status Monitor for S+S of aspiration, bronchospasm, excess secretions Digitalis Digoxin Immune Fab Digoxin: (PO/IV) (Ovine, Digibind) Take apical pulse for 60sec. If < 60 BPM hold Route: IV digoxin dose and contact prescriber. Monitor serum digoxin (it has a narrow therapeutic index), potassium, magnesium, calcium. Monitor for S+S toxicity: anorexia, nausea, vomiting, diarrhea, visual disturbances, cardiac arrhythmias Digoxin Immune Fab: Skin allergy testing prior to administration for history of allergy or previous therapy of this drug Monitor cardiac status + rhythm, neurological status Toxicity reversal within an hour (adults), minutes (children) of antidote administration Monitor serum potassium, critical within first few hours; serum digoxin levels, ECG for 2-3 weeks post administration Heparin Protamine sulfate Heparin: (IV/SC) (IV) Monitor for spontaneous bleeding, thrombocytopenia Monitor aPTT levels Protamine Sulfate: Sudden drop in BP Monitor BP+ P q15-30 min Monitor aPTT Opioid Naloxone (IV-adults, Opioids: -

Effect of Sodium Nitrite, Sodium Erythorbate and Organic Acid Salts on Germination and Outgrowth of Clostridium Perfringens Spores in Ham During Abusive Cooling
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Dissertations, Theses, & Student Research in Food Science and Technology Food Science and Technology Department Fall 9-19-2011 Effect of Sodium Nitrite, Sodium Erythorbate and Organic Acid Salts on Germination and Outgrowth of Clostridium perfringens Spores in Ham during Abusive Cooling Mauricio A. Redondo University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/foodscidiss Part of the Food Chemistry Commons, Food Microbiology Commons, and the Food Processing Commons Redondo, Mauricio A., "Effect of Sodium Nitrite, Sodium Erythorbate and Organic Acid Salts on Germination and Outgrowth of Clostridium perfringens Spores in Ham during Abusive Cooling" (2011). Dissertations, Theses, & Student Research in Food Science and Technology. 18. https://digitalcommons.unl.edu/foodscidiss/18 This Article is brought to you for free and open access by the Food Science and Technology Department at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Dissertations, Theses, & Student Research in Food Science and Technology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. EFFECT OF SODIUM NITRITE, SODIUM ERYTHORBATE AND ORGANIC ACID SALTS ON GERMINATION AND OUTGROWTH OF CLOSTRIDIUM PERFRINGENS SPORES IN HAM DURING ABUSIVE COOLING By Mauricio Redondo-Solano A THESIS Presented to the Faculty of The Graduate College at the University of Nebraska In Partial Fulfillment of Requirements For the Degree of Master of Science Major: Food Science and Technology Under the supervision of Professor Harshavardhan Thippareddi Lincoln, Nebraska September, 2011 EFFECT OF SODIUM NITRITE, SODIUM ERYTHORBATE AND ORGANIC ACID SALTS ON GERMINATION AND OUTGROWTH OF CLOSTRIDIUM PERFRINGENS SPORES IN HAM DURING ABUSIVE COOLING Mauricio Redondo-Solano, M. -

Product Information
PRODUCT INFORMATION DIGIFAB®1 POWDER FOR INJECTION (DIGOXIN-SPECIFIC ANTIBODY FRAGMENT F(Ab) (OVINE)) 1 NAME OF THE MEDICINE Digoxin-specific antibody fragment F(Ab) (Ovine) 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial of DIGIFAB Powder for Injection, will bind approximately 0.5 mg digoxin, contains 40 mg of digoxin- specific antibody fragment F(Ab). These fragments are obtained from blood of healthy sheep immunized with a digoxin derivative, digoxin- dicarboxymethoxylamine (DDMA), a digoxin analogue which contains the functionally essential cyclopentaperhydrophenanthrene:lactone ring moiety coupled to keyhole limpet hemocyanin (KLH). The final product is prepared by isolating the immunoglobulin fraction of the ovine serum, digesting it with papain and isolating the digoxin-specific Fab fragments by affinity chromatography. For the full list of excipients, see Section 6.1 List of Excipients. 3 PHARMACEUTICAL FORM DIGIFAB, digoxin-specific antibody fragment F(Ab) (Ovine) Powder for Injection, is a sterile, purified, lyophilized off-white powder of digoxin-specific antibody ovine Fab (monovalent) immunoglobulin fragments. The pH of the reconstituted solution is in between 4.5-5.5. The product is intended for intravenous administration after reconstitution with 4 mL of Sterile Water for Injection. 4 CLINICAL PARTICULARS 4.1 THERAPEUTIC INDICATIONS Digoxin-specific antibody fragment F(Ab) (Ovine) DIGIFAB is indicated for the treatment of known (or strongly suspected) life-threatening digoxin toxicity associated with ventricular arrhythmias, progressive bradycardia, or second or third degree heart block not responsive to atropine, and where additional measures besides withdrawal of digoxin and correction of serum electrolyte abnormalities are considered necessary. Consequences of multiple dosing with DIGIFAB have not been evaluated. -

Ingestions, Intoxications, and the Critically Ill Child Poisoning in Children
Ingestions, Intoxications, and the Critically Ill Child Poisoning in Children • 1 million cases of exposure to toxins in children younger than 6 years reported in the U.S. In 1993 • estimated that another 1 million exposures to toxins not reported • 1% have moderate or major life-threatening symptoms • 60-100 deaths annually in the U.S Poisoning in Children Less Than 5 Years Old • accounts for 85-90% of pediatric poisoning • is generally accidental • secondary to exploratory behavior and lack of supervision • tend to involve single agent ingestions Poisoning in Children Over 5 Years Old • accounts for 10-15% of pediatric poisoning • is generally intentional • secondary to suicide attempts or gestures, or to intoxications and inadvertent overdose • tend to involve multiple agent ingestions General Concepts for Pediatric Poisoning • Prevention • Initial Stabilization • Diagnosis • Specific Antidotes Management of the Poisoned Child • Treat the Patient, Not the Poison --patient-specific treatment is safer, less expensive, and more effective Management of the Poisoned Child • Stabilization --Airway --Breathing --Circulation --Disability (neurologic) Management of the Poisoned Child • Respiratory Failure --airway obstruction from secretions, refluxed gastric contents, airway muscle relaxation --respiratory muscle rigidity --loss of respiratory drive --pulmonary edema Management of the Poisoned Child • Cardiovascular Collapse --arteriolar dilation --venous dilation --myocardial depression --dysrhythmias Management of the Poisoned Child • Neurologic -

Antidote List
Antidote List Recommended minimum amounts for hospital pharmacies to stock Call the Maryland Poison Center for expert advice on the treatment of all poisonings and overdoses: 1-800-222-1222 Poisoning Minimum Stocking Antidote Indication Recommendations Oral: 120 grams Acetylcysteine Acetaminophen IV: 96 grams Crotaline snake Antivenin, snake (CroFab®) 12 vials envenomation 1 vial Black widow spider (Note: Merck limits distribution Antivenin, black widow spider envenomation to cases of confirmed bites with symptoms only) Organophosphate & Atropine carbamate insecticides, nerve 165 mg gases Calcium chloride Calcium channel blockers 10 grams 2.25 grams Calcium disodium EDTA Lead (Available direct from ASD Specialty Healthcare) Hydrofluoric acid 1 kg (powder) Calcium gluconate Calcium channel blockers IV: 30 grams 1 kit Cyanide Antidote Kit Cyanide (or Hydroxocobalamin, see below) Cyproheptadine (Periactin®) Serotonin syndrome 80 mg Neuroleptic malignant Dantrolene syndrome, stimulant-induced 720 mg hyperthermia Deferoxamine mesylate Iron 8 grams (Desferal®) Digoxin Immune FAB Digoxin 20 vials (Digibind®, DigiFab®) Dimercaprol (BAL) Heavy metals 1.5 grams 1 | P a g e Maryland Poison Center Antidote List – continued Poisoning Minimum Stocking Antidote Indication Recommendations DMSA (Succimer, Chemet®) Heavy metals 2000 mg Folic acid Methanol IV: 150 mg Flumazenil (Romazicon®) Benzodiazepines 10 mg Fomepizole (Antizol®) Ethylene glycol, methanol 12 grams Beta blockers, Glucagon 50 mg calcium channel blockers Hydroxocobalamin (Cyanokit®) Cyanide