Antidotes Atropine Calcium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

EH&S COVID-19 Chemical Disinfectant Safety Information
COVID-19 CHEMICAL DISINFECTANT SAFETY INFORMATION Updated June 24, 2020 The COVID-19 pandemic has caused an increase in the number of disinfection products used throughout UW departments. This document provides general information about EPA-registered disinfectants, such as potential health hazards and personal protective equipment recommendations, for the commonly used disinfectants at the UW. Chemical Disinfectant Base / Category Products Potential Hazards Controls ● Ethyl alcohol Highly flammable and could form explosive Disposable nitrile gloves Alcohols ● ● vapor/air mixtures. ● Use in well-ventilated areas away from o Clorox 4 in One Disinfecting Spray Ready-to-Use ● May react violently with strong oxidants. ignition sources ● Alcohols may de-fat the skin and cause ● Wear long sleeve shirt and pants ● Isopropyl alcohol dermatitis. ● Closed toe shoes o Isopropyl Alcohol Antiseptic ● Inhalation of concentrated alcohol vapor 75% Topical Solution, MM may cause irritation of the respiratory tract (Ready to Use) and effects on the central nervous system. o Opti-Cide Surface Wipes o Powell PII Disinfectant Wipes o Super Sani Cloth Germicidal Wipe 201 Hall Health Center, Box 354400, Seattle, WA 98195-4400 206.543.7262 ᅵ fax 206.543.3351ᅵ www.ehs.washington.edu ● Formaldehyde Formaldehyde in gas form is extremely Disposable nitrile gloves for Aldehydes ● ● flammable. It forms explosive mixtures with concentrations 10% or less ● Paraformaldehyde air. ● Medium or heavyweight nitrile, neoprene, ● Glutaraldehyde ● It should only be used in well-ventilated natural rubber, or PVC gloves for ● Ortho-phthalaldehyde (OPA) areas. concentrated solutions ● The chemicals are irritating, toxic to humans ● Protective clothing to minimize skin upon contact or inhalation of high contact concentrations. -

IV-23 N. Hydroxocobalamin (Cyanokit®)
N. Hydroxocobalamin (Cyanokit®) I. Classification •Cyanide antidote II. Actions •Binds cyanide ions with more affinity than hemoglobin molecule •Cyanide ion and hydroxocobalamin form cyanocobalamin (Vitamin B12) which is then excreted in the urine. III. Indications •Known or suspected cyanide poisoning - patients at high risk (industrial accidents, fire victims with smoke inhalation, known overdose, etc) with one more more of the following symptoms: - Altered mental status, confusion, seizures, coma - Headache - Chest pain or tightness - Shortness of breath, bradypnea, tachypnea - Hypertension (early), hypotension (late), cardiovascular collapse - Nausea, vomiting - Cardiac arrest - Mydriasis (dilated pupils) IV. Contraindications •Known allergic reaction to hydroxocobalamin or cyanocobalamin V. Adverse effects A. Cardiovascular •Ventricular extrasystoles •Tachycardia •Transient hypertension B. Neurological •Memory impairment •Dizziness •Restlessness C. Respiratory •Dyspnea •Dry Throat •Throat tightness D. Gastrointestinal •Abdominal discomfort •Dysphagia •Vomiting, diarrhea •Hematochezia E. General •Allergic reaction, pruritis, anaphylaxis •Hot flush SUBJECT : PATIENT CARE GUIDELINES AND STANDING ORDERS FOR BLS AND ALS UNITS REFERENCE NO. III-01 PUBLICATION : 1/11/21 IV-23 VI. Administration A. Do not administer hydroxocobalamin through the same IV site/set as the following medications: dopamine, fentanyl, dobutamine, diazepam, nitroglycerin, pentobarbital, propofol, thiopental, sodium thiosulfate, sodium nitrite and ascorbic acid. You must start a second IV to administer hydroxocobalamin in patient’s receiving these medications. B. Adult: •5 grams IV/IO over 15 minutes (Stocked as single 5gm bottle, or two 2.5 gm bottles) -Single 5 gm Bottle - Reconstituted with 200 ml Normal Saline. -Two 2.5 gm Bottles, give over 7.5 minutes each. - Each vial reconstituted with 100 ml Normal Saline. C. Pediatric •100 mg/kg IV/IO of hydroxocobalamin (reconstituted with Normal Saline the same as adults) over 15 minutes. -

Review of Succimer for Treatment of Lead Poisoning
Review of Succimer for treatment of lead poisoning Glyn N Volans MD, BSc, FRCP. Department of Clinical Pharmacology, School of Medicine at Guy's, King's College & St Thomas' Hospitals, St Thomas' Hospital, London, UK Lakshman Karalliedde MB BS, DA, FRCA Consultant Medical Toxicologist, CHaPD (London), Health Protection Agency UK, Visiting Senior Lecturer, Division of Public Health Sciences, King's College Medical School, King's College , London Senior Research Collaborator, South Asian Clinical Toxicology Research Collaboration, Faculty of Medicine, Peradeniya, Sri Lanka. Heather M Wiseman BSc MSc Medical Toxicology Information Services, Guy’s and St Thomas’ NHS Foundation Trust, London SE1 9RT, UK. Contact details: Heather Wiseman Medical Toxicology Information Services Guy’s & St Thomas’ NHS Foundation Trust Mary Sheridan House Guy’s Hospital Great Maze Pond London SE1 9RT Tel 020 7188 7188 extn 51699 or 020 7188 0600 (admin office) Date 10th March 2010 succimer V 29 Nov 10.doc last saved: 29-Nov-10 11:30 Page 1 of 50 CONTENTS 1 Summary 2. Name of the focal point in WHO submitting or supporting the application 3. Name of the organization(s) consulted and/or supporting the application 4. International Nonproprietary Name (INN, generic name) of the medicine 5. Formulation proposed for inclusion 6. International availability 7. Whether listing is requested as an individual medicine or as an example of a therapeutic group 8. Public health relevance 8.1 Epidemiological information on burden of disease due to lead poisoning 8.2 Assessment of current use 8.2.1 Treatment of children with lead poisoning 8.2.2 Other indications 9. -

Sodium Cellulose Phosphate Sodium Edetate
Sevelamer/Sodium Edetate 1463 excreted by the kidneys. It may be used as a diagnostic is not less than 9.5% and not more than 13.0%, all calculated on supplement should not be given simultaneously with test for lead poisoning but measurement of blood-lead the dried basis. The calcium binding capacity, calculated on the sodium cellulose phosphate. dried basis, is not less than 1.8 mmol per g. concentrations is generally preferred. Sodium cellulose phosphate has also been used for the Sodium calcium edetate is also a chelator of other Adverse Effects and Precautions investigation of calcium absorption. heavy-metal polyvalent ions, including chromium. A Diarrhoea and other gastrointestinal disturbances have Preparations cream containing sodium calcium edetate 10% has been reported. USP 31: Cellulose Sodium Phosphate for Oral Suspension. been used in the treatment of chrome ulcers and skin Sodium cellulose phosphate should not be given to pa- Proprietary Preparations (details are given in Part 3) sensitivity reactions due to contact with heavy metals. tients with primary or secondary hyperparathyroidism, Spain: Anacalcit; USA: Calcibind. Sodium calcium edetate is also used as a pharmaceuti- hypomagnesaemia, hypocalcaemia, bone disease, or cal excipient and as a food additive. enteric hyperoxaluria. It should be used cautiously in pregnant women and children, since they have high Sodium Edetate In the treatment of lead poisoning, sodium calcium calcium requirements. Sodu edetynian. edetate may be given by intramuscular injection or by Patients should be monitored for electrolyte distur- intravenous infusion. The intramuscular route may be Эдетат Натрия bances. Uptake of sodium and phosphate may increase CAS — 17421-79-3 (monosodium edetate). -

Uses and Administration Adverse Effects and Precautions Pharmacokinetics Uses and Administration
1549 The adverse effects of dicobalt edetate are more severe in year-old child: case report and review of literature. Z Kardiol 2005; 94: References. 817-2 3. the absence of cyanide. Therefore, dicobalt edetate should I. Schaumann W, et a!. Kinetics of the Fab fragments of digoxin antibodies and of bound digoxin in patients with severe digoxin intoxication. Eur 1 not be given unless cyanide poisoning is confirmed and 30: Administration in renal impairment. In renal impairment, Clin Pharmacol 1986; 527-33. poisoning is severe such as when consciousness is impaired. 2. Ujhelyi MR, Robert S. Pharmacokinetic aspects of digoxin-specific Fab elimination of antibody-bound digoxin or digitoxin is therapy in the management of digitalis toxicity. Clin Pharmacokinet 1995; Oedema. A patient with cyanide toxicity developed delayed1·3 and antibody fragments can be detected in the 28: 483-93. plasma for 2 to 3 weeks after treatment.1 The rebound in 3. Renard C, et al. Pharmacokinetics of dig,'><i<1-sp,eei1ie Fab: effects of severe facial and pulmonary oedema after treatment with decreased renal function and age. 1997; 44: 135-8. dicobalt edetate.1 It has been suggested that when dicobalt free-digoxin concentrations that has been reported after edetate is used, facilities for intubation and resuscitation treatment with digoxin-specific antibody fragments (see P (Jrations should be immediately available. Poisoning, below), occurred much later in patients with r.�p renal impairment than in those with normal renal func Proprietary Preparations (details are given in Volume B) 1. Dodds C, McKnight C. Cyanide toxicity after immersion and the hazards tion. -

Cyanocobalamin-A Case for Withdrawal
686 Journal of the Royal Society of Medicine Volume 85 November 1992 Cyanocobalamin- a case for withdrawal: discussion paper A G Freeman MD FRCP Meadow Rise, 3 Lakeside, Swindon SN3 IQE Keywords: anaemia, pernicious; optic neuropathies; chronic cyanide intoxication; hydroxocobalamin; cyanocobalamin It seems evident that controversy still surrounds the reduced ability to detoxify the cyanide in the tobacco- treatment of pernicious anaemia and other vitamin smoke to which they are exposed'0. B12 deficiency disorders. The long quest for the 'anti- Patients with tobacco amblyopia who have normal pernicious anaemia factor' in the liver seemed to serum vitamin B12 levels need not continue therapy have ended in 1948 when pure cyanocobalamin was with intramuscular hydroxocobalamin once their isolated. This was found to be very active thera- visual acuity and visual fields have returned to peutically when given by intramuscular injection and normal providing they abstain from further smoking. was non-toxic in extremely high doses'. However, those patients who have low serum vitamin Lederle, in a recent commentary2, advocates that B12 levels or evidence of -defective vitamin B12 patients with pernicious anaemia should now be absorption will need to continue-indefinitely with treated with oral cyanocobalamin. He is not without hydroxocobalamin irrespective of their smoking support in that 40% of patients with pernicious habits as will all patients with pernicious anaemia anaemia in Sweden are being similarly treated3. and other vitamin B12 deficiency disorders who are He further states that such!- treatment is cheap at risk of developing- optic neuropathy if they and effective, produces clinical and haematological are smokers. -

Thiosulfate De Sodium Sterop
Thiosulfate de Sodium Sterop 1. DENOMINATION DU MEDICAMENT THIOSULFATE DE SODIUM STEROP 1g/5ml solution injectable 2. COMPOSITION QUALITATIVE ET QUANTITATIVE Substance active : Une ampoule de 5ml contient 1g de thiosulfate de sodium dans de l’eau pour préparations injectables. 1 ml de solution contient 200mg de thiosulfate de sodium. Pour la liste complète des excipients, voir rubrique 6.1. 3. FORME PHARMACEUTIQUE Solution injectable. 4. DONNEES CLINIQUES 4.1 Indications thérapeutiques - Antidote dans le traitement des intoxications par les cyanures ainsi que par le nitroprussiate de sodium. - Prévention des effets néphrotoxiques du cisplatine. Thiosulfate de Sodium Sterop © Pharma.be Pagina 1 van 6 4.2 Posologie et mode d’administration Mode d'administration Pour voie intraveineuse lente. Posologie Intoxications par les cyanures: Adultes: La posologie habituelle est de 12,5 g de thiosulfate de sodium, administré par voie I.V. sur une période de 5 à 10 minutes. Enfants: La posologie habituelle est de 412,5 mg de thiosulfate de sodium par kg, administré par voie I.V. à la vitesse de 0,625 à 1,25 g/minute sur une période de 5 à 10 minutes. Si les symptômes persistent ou réapparaissent dans la demi-heure ou dans l’heure ayant suivi la première injection, une seconde injection de thiosulfate de sodium est nécessaire avec comme posologie la moitié de celle de la première injection. En prévention des effets néphrotoxiques du cisplatine: Chez l'adulte, la posologie initiale est de 9 g de thiosulfate de sodium par m2 de surface corporelle, administré par voie I.V. Elle est suivie par l'injection de 1,2 g de thiosulfate de sodium par m2 de surface corporelle par heure, en perfusion I.V. -
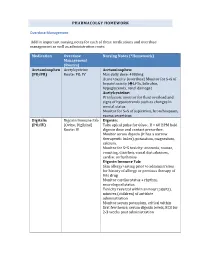
PHARMACOLGY HOMEWORK Overdose Management Add In
PHARMACOLGY HOMEWORK Overdose Management Add in important nursing notes for each of these medications and overdose management as well as administration route. Medication Overdose Nursing Notes (*Homework) Management (Routes) Acetaminophen Acetylcysteine Acetaminophen: (PO/PR) Route: PO, IV Max daily dose: 4000mg Acute toxicity (overdose) Monitor for S+S of hepatotoxicity (éLFTs, bilirubin, hypoglycemia, renal damage) Acetylcysteine: IV infusion: monitor for fluid overload and signs of hyponatremia such as changes in mental status Monitor for S+S of aspiration, bronchospasm, excess secretions Digitalis Digoxin Immune Fab Digoxin: (PO/IV) (Ovine, Digibind) Take apical pulse for 60sec. If < 60 BPM hold Route: IV digoxin dose and contact prescriber. Monitor serum digoxin (it has a narrow therapeutic index), potassium, magnesium, calcium. Monitor for S+S toxicity: anorexia, nausea, vomiting, diarrhea, visual disturbances, cardiac arrhythmias Digoxin Immune Fab: Skin allergy testing prior to administration for history of allergy or previous therapy of this drug Monitor cardiac status + rhythm, neurological status Toxicity reversal within an hour (adults), minutes (children) of antidote administration Monitor serum potassium, critical within first few hours; serum digoxin levels, ECG for 2-3 weeks post administration Heparin Protamine sulfate Heparin: (IV/SC) (IV) Monitor for spontaneous bleeding, thrombocytopenia Monitor aPTT levels Protamine Sulfate: Sudden drop in BP Monitor BP+ P q15-30 min Monitor aPTT Opioid Naloxone (IV-adults, Opioids: -

Expert Consensus Guidelines for Stocking of Antidotes in Hospitals That Provide Emergency Care
TOXICOLOGY/CONCEPTS Expert Consensus Guidelines for Stocking of Antidotes in Hospitals That Provide Emergency Care Richard C. Dart, MD, PhD From the Rocky Mountain Poison & Drug Center - Denver Health, Denver, CO (Dart, Heard, Schaeffer, Stephen W. Borron, MD, MS Bogdan, Alhelail, Buchanan, Hoppe, Lavonas, Mlynarchek, Phua, Rhyee, Varney, Zosel); Department of E. Martin Caravati, MD, MPH Surgery, University of Texas Health Sciences Center, San Antonio, TX (Borron); Division of Emergency Daniel J. Cobaugh, PharmD Medicine, Utah Poison Control Center, University of Utah Health Sciences Center, Salt Lake City, UT Steven C. Curry, MD (Caravati); ASHP Research and Education Foundation, Bethesda, MD (Cobaugh); Department of Jay L. Falk, MD Medical Toxicology and Banner Poison Control Center, Banner Good Samaritan Medical Center, Lewis Goldfrank, MD Phoenix, AZ (Curry); Department of Emergency Medicine, Orlando Regional Medical Center, University of Susan E. Gorman, PharmD, MS Florida, Orlando, FL (Falk); New York City Poison Center; New York University School of Medicine, New Stephen Groft, PharmD York, NY (Goldfrank); Division of Strategic National Stockpile, Centers for Disease Control and Kennon Heard, MD Prevention, Atlanta, GA (Gorman); Office of Rare Diseases Research, Bethesda, MD (Groft); Division of Ken Miller, MD, PhD Emergency Medicine, School of Medicine (Dart, Heard, Lavonas, Schaeffer) and Department of Kent R. Olson, MD Pharmaceutical Sciences, School of Pharmacy (Bogdan), University of Colorado Denver, Aurora, CO; Gerald O’Malley, DO Orange County Fire Authority and Orange County Health Care Agency Emergency Medical Services, Donna Seger, MD Irvine, CA, and National Association of EMS Physicians, Lenexa, KS, (Miller); University of California, Steven A. Seifert, MD San Francisco, and San Francisco Division, California Poison Control System, San Francisco, CA Marco L. -

Reactions of Alcohols & Ethers 1
REACTIONS OF ALCOHOLS & ETHERS 1. Combustion (Extreme Oxidation) alcohol + oxygen carbon dioxide + water 2 CH3CH2OH + 6 O2 4 CO2 + 6 H2O 2. Elimination (Dehydration) ° alcohol H 2 S O 4/ 1 0 0 C alkene + water H2SO4/100 ° C CH3CH2CH2OH CH3CH=CH2 + H2O 3. Condensation ° excess alcohol H 2 S O 4 / 1 4 0 C ether + water H2SO4/140 ° C 2 CH3CH2OH CH3CH2OCH2CH3 + H2O 4. Substitution Lucas Reagent alcohol + hydrogen halide Z n C l2 alkyl halide + water ZnCl2 CH3CH2OH + HCl CH3CH2Cl + H2O • This reaction with the Lucas Reagent (ZnCl2) is a qualitative test for the different types of alcohols because the rate of the reaction differs greatly for a primary, secondary and tertiary alcohol. • The difference in rates is due to the solubility of the resulting alkyl halides • Tertiary Alcohol→ turns cloudy immediately (the alkyl halide is not soluble in water and precipitates out) • Secondary Alcohol → turns cloudy after 5 minutes • Primary Alcohol → takes much longer than 5 minutes to turn cloudy 5. Oxidation • Uses an oxidizing agent such as potassium permanganate (KMnO4) or potassium dichromate (K2Cr2O7). • This reaction can also be used as a qualitative test for the different types of alcohols because there is a distinct colour change. dichromate → chromium 3+ (orange) → (green) permanganate → manganese (IV) oxide (purple) → (brown) Tertiary Alcohol not oxidized under normal conditions CH3 KMnO4 H3C C OH NO REACTION K2Cr2O7 CH3 tertbutyl alcohol Secondary Alcohol ketone + hydrogen ions H O KMnO4 H3C C CH3 + K2Cr2O7 C + 2 H H3C CH3 OH propanone 2-propanol Primary Alcohol aldehyde + water carboxylic acid + hydrogen ions O KMnO4 O H H KMnO H H 4 + CH3CH2CH2OH C C C H C C C H + 2 H K2Cr2O7 + H2O K Cr O H H H 2 2 7 HO H H 1-propanol propanal propanoic acid 6. -

Chemical Disinfectants | Disinfection & Sterilization Guidelines | Guidelines Library | Infection Control | CDC 3/19/20, 14:52
Chemical Disinfectants | Disinfection & Sterilization Guidelines | Guidelines Library | Infection Control | CDC 3/19/20, 14:52 Infection Control Chemical Disinfectants Guideline for Disinfection and Sterilization in Healthcare Facilities (2008) Alcohol Overview. In the healthcare setting, “alcohol” refers to two water-soluble chemical compounds—ethyl alcohol and isopropyl alcohol —that have generally underrated germicidal characteristics 482. FDA has not cleared any liquid chemical sterilant or high- level disinfectant with alcohol as the main active ingredient. These alcohols are rapidly bactericidal rather than bacteriostatic against vegetative forms of bacteria; they also are tuberculocidal, fungicidal, and virucidal but do not destroy bacterial spores. Their cidal activity drops sharply when diluted below 50% concentration, and the optimum bactericidal concentration is 60%–90% solutions in water (volume/volume) 483, 484. Mode of Action. The most feasible explanation for the antimicrobial action of alcohol is denaturation of proteins. This mechanism is supported by the observation that absolute ethyl alcohol, a dehydrating agent, is less bactericidal than mixtures of alcohol and water because proteins are denatured more quickly in the presence of water 484, 485. Protein denaturation also is consistent with observations that alcohol destroys the dehydrogenases of Escherichia coli 486, and that ethyl alcohol increases the lag phase of Enterobacter aerogenes 487 and that the lag phase e!ect could be reversed by adding certain amino acids. The bacteriostatic action was believed caused by inhibition of the production of metabolites essential for rapid cell division. Microbicidal Activity. Methyl alcohol (methanol) has the weakest bactericidal action of the alcohols and thus seldom is used in healthcare 488. The bactericidal activity of various concentrations of ethyl alcohol (ethanol) was examined against a variety of microorganisms in exposure periods ranging from 10 seconds to 1 hour 483. -

Adult BLS Standing Orders • Ensure EMS Provider Safety, Consider HAZMAT Activation
Imperial County Public Health Department Emergency Medical Services Agency Policy/Procedure/Protocol Manual Treatment Protocols Date: 07/01/2021 Poisoning/Intoxication/Envenomation - Adult Policy #9160A Adult BLS Standing Orders • Ensure EMS provider safety, consider HAZMAT activation. Recognize, Notify, Isolate • Universal Patient Protocol • Do not approach patient or location if scene safety is in question • Obtain accurate history of incident: o Name of product or substance o Quantity ingested, and/or duration of exposure o Time elapsed since exposure o If safe and accessible, bring medications or bottles to hospital • Move victim(s) to safe environment • Externally decontaminate - PRN • Continuously monitor ECG, blood pressure, pulse oximetry, and capnography (if ALS present) PRN • Give oxygen and provide airway support per Airway Policy • Contact Poison Control Center as needed 1 (800) 222-1222 Suspected Opioid Overdose with Respirations <12 RPM • If possible, avoid the use of a supraglottic device prior to the administration of naloxone • Administer naloxone 0.1 mg/kg, max of 2 mg IN. May repeat up to three (3) times, q5min • May assist family/friends on-scene with administration of patient’s own naloxone • NOTE - Use with caution in opioid dependent pain management patients • Assess vitals, with specific attention to respiratory rate and respiratory drive • Note pupil exam • Note drug paraphernalia or medication bottles near patient Suspected Stimulant Overdose with Sudden Hypoventilation, Oxygen Desaturation, or Apnea • High flow