Calcium Gluconate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Management of Poisoning
MOH CLINICAL PRACTICE GUIDELINES December/2011 Management of Poisoning Health Ministry of Sciences Chapter of Emergency College of College of Family Manpower Authority Physicians Physicians, Physicians Academy of Medicine, Singapore Singapore Singapore Singapore Medical Pharmaceutical Society Society for Emergency Toxicology Singapore Paediatric Association of Singapore Medicine in Singapore Society (Singapore) Society Executive summary of recommendations Details of recommendations can be found in the main text at the pages indicated. Principles of management of acute poisoning – resuscitating the poisoned patient GPP In a critically poisoned patient, measures beyond standard resuscitative protocol like those listed above need to be implemented and a specialist experienced in poisoning management should be consulted (pg 55). GPP D Prolonged resuscitation should be attempted in drug-induced cardiac arrest (pg 55). Grade D, Level 3 1 C Titrated doses of naloxone, together with bag-valve-mask ventilation, should be administered for suspected opioid-induced coma, prior to intubation for respiratory insuffi ciency (pg 56). Grade C, Level 2+ D In bradycardia due to calcium channel or beta-blocker toxicity that is refractory to conventional vasopressor therapy, intravenous calcium, glucagon or insulin should be used (pg 57). Grade D, Level 3 B Patients with actual or potential life threatening cardiac arrhythmia, hyperkalaemia or rapidly progressive toxicity from digoxin poisoning should be treated with digoxin-specifi c antibodies (pg 57). Grade B, Level 2++ B Titrated doses of benzodiazepine should be given in hyperadrenergic- induced tachycardia states resulting from poisoning (pg 57). Grade B, Level 1+ D Non-selective beta-blockers, like propranolol, should be avoided in stimulant toxicity as unopposed alpha agonism may worsen accompanying hypertension (pg 57). -

GHS Calcium Lactate Gluconate MSDS.Pdf
Safety Data Sheet (Calcium Lactate Gluconate) DATE PREPARED: 7/9/2015 Section 1. Product and Company Identification Product Name Calcium Lactate Gluconate CAS Number 11116-97-5 Parchem - fine & specialty chemicals EMERGENCY RESPONSE NUMBER 415 Huguenot Street CHEMTEL New Rochelle, NY 10801 Toll Free US & Canada: 1 (800) 255-3924 (914) 654-6800 (914) 654-6899 All other Origins: 1 (813) 248-0585 parchem.com [email protected] Collect Calls Accepted Section 2. Hazards Identification Classification of the substance or mixture Classification according to Directive 67/548/EEC or 1999/45/EC as amended: This preparation does not meet the criteria for classification according to Directive 1999/45/EC as amended. Classification according to Regulation (EC) No 1272/2008 as amended: This mixture does not meet the criteria for classification according to Regulation (EC) 1272/2008 as amended. Hazard and precautionary statements Hazard statements: The substance does not meet the criteria for classification. Precautionary statements: Not available. Appearance & Odor: White powder with no odor. Fire & Explosion Hazards Potential for dust explosion may exist. This product is not defined as flammable or combustible. However, the product may decompose under fire conditions to produce toxic oxides of carbon. Depending upon conditions, dusts may be sensitive to static discharge. Avoid possibility of dry powder and friction causing static electricity in presence of flammables (See NFPA-77, Chpt. 6) Primary Route of Exposure: Skin and eye contact are the primary routes of exposure to this product. Inhalation Acute Exposure: Inhalation of dust may cause mild irritation. Skin Contact - Acute: Skin contact is not expected to cause irritation. -

Wednesday May 26, 1999
5±26±99 Vol. 64 No. 101 Wednesday Pages 28333±28712 May 26, 1999 federal register 1 VerDate 06-MAY-99 21:29 May 25, 1999 Jkt 183247 PO 00000 Frm 00001 Fmt 4710 Sfmt 4710 E:\FR\FM\26MYWS.XXX pfrm03 PsN: 26MYWS II Federal Register / Vol. 64, No. 101 / Wednesday, May 26, 1999 The FEDERAL REGISTER is published daily, Monday through SUBSCRIPTIONS AND COPIES Friday, except official holidays, by the Office of the Federal Register, National Archives and Records Administration, PUBLIC Washington, DC 20408, under the Federal Register Act (44 U.S.C. Subscriptions: Ch. 15) and the regulations of the Administrative Committee of Paper or fiche 202±512±1800 the Federal Register (1 CFR Ch. I). The Superintendent of Assistance with public subscriptions 512±1806 Documents, U.S. Government Printing Office, Washington, DC 20402 is the exclusive distributor of the official edition. General online information 202±512±1530; 1±888±293±6498 Single copies/back copies: The Federal Register provides a uniform system for making available to the public regulations and legal notices issued by Paper or fiche 512±1800 Federal agencies. These include Presidential proclamations and Assistance with public single copies 512±1803 Executive Orders, Federal agency documents having general FEDERAL AGENCIES applicability and legal effect, documents required to be published Subscriptions: by act of Congress, and other Federal agency documents of public Paper or fiche 523±5243 interest. Assistance with Federal agency subscriptions 523±5243 Documents are on file for public inspection in the Office of the Federal Register the day before they are published, unless the issuing agency requests earlier filing. -

Magnesium Sulfate for Neuroprotection Practice Guideline
[Type text] [Type text] Updated June 2013 Magnesium Sulfate for Neuroprotection Practice Guideline I. Background: Magnesium sulfate has been suggested to have neuro-protective effect in retrospective studies from 1987 and 1996. Since that time three randomized control trials have been performed to assess magnesium therapy for fetal neuroprotection. These studies have failed to demonstrate statistically significant decrease in combined outcome of cerebral palsy and death or improved overall neonatal survival. However, these results did demonstrate a significant decrease in cerebral palsy of any severity by 30%, particularly moderate-severe cerebral palsy (40-45%). The number needed to treat at less than 32 weeks gestation is 56. The presumptive mechanism of action for magnesium sulfate focuses on the N-methyl-D-aspartate receptor. Additional magnesium effects include calcium channel blockade resulting in cerebrovascular relaxation and magnesium mediated decreases in free radical production and reductions in the production of inflammatory cytokines. Magnesium sulfate should not be used as a tocolytic simply because of the potential for neuro-protective effects. In a recent committee opinion, ACOG states “the available evidence suggests that magnesium sulfate given before anticipated early preterm birth reduces the risk of cerebral palsy in surviving infants” but specific guidelines should be established. “The U.S. FDA has recently changed the classification of magnesium sulfate injection from Category A to Category D. However, this change was based on a small number of neonatal outcomes in cases in which the average duration of exposure was 9.6 weeks. The ACOG Committee on Obstetric Practice and the Society for Maternal-Fetal Medicine continue to support the use of magnesium sulfate in obstetric care for appropriate conditions and for appropriate, short term (usually less than 48 hours) durations of treatment.” II. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Magnesium Sulfate
MODULE III MAGNESIUM SULFATE Manual for Procurement & Supply of Quality-Assured MNCH Commodities MAGNESIUM SULFATE INJECTION, 500 MG/ML IN 2-ML AND 10-ML AMPOULE GENERAL PRODUCT INFORMATION Pre-eclampsia and eclampsia is the second-leading cause of maternal death in low- and middle-income countries. It is most often detected through the elevation of blood pressure during pregnancy, which can be followed by seizures, kidney and liver damage, and maternal and fetal death, if untreated. Magnesium sulfate is recognized by WHO as the safest, most effective, and lowest-cost medicine for treating pre-eclampsia and eclampsia. It is also considered an essential medicine by the UN Commission on Life-Saving Commodities for Women and Children. Other anticonvulsant medicines, such as diazepam and phenytoin, are less effective and riskier. Magnesium sulfate should be the sole first-line treatment of pre-eclampsia and eclampsia that should be procured over other anticonvulsants and made available in all health facilities to help lower maternal death rates and improve overall maternal health. MSꟷ1 | Manual for Procurement & Supply of Quality-Assured MNCH Commodities Magnesium Sulfate KEY CONSIDERATIONS IN PROCUREMENT Procurement should be made from trusted sources. This includes manufacturers prequalified by WHO or approved by a SRA for magnesium sulfate injection and 1. those with a proven record of quality products. Procurers need to focus on product quality to ensure that it is sterile and safe for patient use as magnesium sulfate is an injectable medicine. 2. KEY QUALITY CONSIDERATIONS Product specification Products that are procured must comply with pharmacopoeial specifications, such as those of the International Pharmacopoeia, US Pharmacopoeia, and British Pharmacopoeia, as detailed in the “Supply” section 4 below. -

Magnesium for Constipation
Magnesium for Constipation What is Magnesium Oxide? Magnesium is a mineral that the body uses to keep the organs functioning, particularly the kidneys, heart, and muscles. Magnesium can be obtained from foods such as green vegetables, nuts, and whole grain products. Though most people maintain adequate magnesium levels on their own, some disorders can lower magnesium levels, such as gastrointestinal disorders like Irritable Bowel Syndrome (IBS). Magnesium helps to increase the amount of water in the intestines, which can help with bowel movements. It may be used as a laxative due to these properties, or as a supplement for magnesium deficiency. What is the dose amount? The maximum dose for Magnesium is 2 grams or 2000 milligrams. You should not take more than 4 tablets or capsules in one day. Magnesium comes in tablets and capsules (500 mg): take orally as directed by your doctor and take with a full 8-ounce glass of liquid. One Tablespoon of Milk of Magnesium is equal to 500 mg. Tablets/Caplets may be taken all at bedtime or separately throughout the day. Side Effects There can be many side effects related to the intake of oral Magnesium. Some of the frequent side effects include diarrhea. You should notify your doctor immediately if you have any of the following severe side effects: black, tarry stools nausea Michigan Bowel Control Program - 1 - slow reflexes vomit that looks like coffee grounds Where can I purchase Magnesium? You can find Magnesium over the counter at most stores that sell supplements and at pharmacies. It typically costs $5 for a 100-count bottle. -
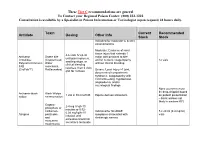
Antidote Stocking Tier C
These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Toxin Current Recommended Antidote Dosing Other Info Stock Stock Indicated for moderate to severe envenomations Moderate: Evidence of local tissue injury that extends 1 4-6 vials IV q2-4h Antivenin Snake bite major joint proximal to bite until pain improves, Crotalidae (Copperhead, and/or numeric coagulopathy 12 vials swelling stops, or Polyvalent Immune Water without clinical bleeding clinical bleeding FAB moccasins, resolves, then 2 vials (CroFab™) Rattlesnakes) Severe: Local injury >1 joint, q6h for 3 doses documented compartment syndrome, coagulopathy with clinical bleeding, hypotension, angioedema, and/or neurological findings None (currently must be drop-shipped based Antivenin-black Black Widow 1 vial in 50 ml of NS Equine derived antivenom on patient presentation widow envenomation – black widows not likely in western KY) Organo- 2-4 mg IV q5-10 phosphate or minutes or 0.02- carbamate Indicated for SLUDGE 5 x 20 ml (0.4 mg/ml) 0.08 mg/kg/hr IV Atropine pesticides symptoms associated with vials infusion until and cholinergic excess excessive bronchial muscarine secretions terminate mushrooms These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Antidote Current Recommended Toxin -

Calcium Gluconate Injection Contains 95 Mg/Ml Calcium Gluconate Monohydrate and 3.0 Mg/Ml Calcium Saccharate
Calcium Gluconate Injection Contains 95 mg/mL Calcium Gluconate Monohydrate and 3.0 mg/mL Calcium Saccharate Consumer Medicine Information What is in this leaflet Before you are given You should not be given Calcium Gluconate Injection if you have been Calcium Gluconate bed ridden for a long time causing This leaflet answers some common the loss of calcium from the bones. questions about Calcium Gluconate Injection Injection. It does not contain all the You should not be given Calcium available information. It does not take Gluconate Injection if you are being the place of talking to your doctor. When you must not be given treated with certain heart drugs such it as digoxin and digitalis. All medicines have risks and benefits. Your doctor has weighed the risks of You should not be given Calcium You should not be given Calcium you being given Calcium Gluconate Gluconate Injection if you have an Gluconate Injection if the solution is Injection against the benefits they allergy to: discoloured, cloudy, turbid, or a expect it will have for you. • any medicine containing calcium precipitate is present. The solution is gluconate normally a clear, colourless liquid. If you have any concerns about being given this medicine, ask your • any of the ingredients listed at the You should not be given Calcium doctor. end of this leaflet. Gluconate Injection if when diluted with another solution it causes the Some of the symptoms of an allergic Keep this leaflet. solution to precipitate, become You may need to read it again. reaction may include: cloudy, turbid, discolour, or particles • shortness of breath are visible. -

Therapeutic Uses of Magnesium MARY P
COMPLEMENTARY AND ALTERNATIVE MEDICINE Therapeutic Uses of Magnesium MARY P. GUERRERA, MD, University of Connecticut School of Medicine, Farmington, Connecticut STELLA LUCIA VOLPE, P,hD University of Pennsylvania School of Nursing, Philadelphia, Pennsylvania JUN JAMES MAO, MD, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania Magnesium is an essential mineral for optimal metabolic function. Research has shown that the mineral content of magnesium in food sources is declining, and that magnesium depletion has been detected in persons with some chronic diseases. This has led to an increased awareness of proper magnesium intake and its potential therapeutic role in a number of medical conditions. Studies have shown the effectiveness of magnesium in eclampsia and preeclampsia, arrhythmia, severe asthma, and migraine. Other areas that have shown promising results include lowering the risk of metabolic syndrome, improving glucose and insulin metabolism, relieving symptoms of dysmenorrhea, and alleviat- ing leg cramps in women who are pregnant. The use of magnesium for constipation and dyspepsia are accepted as standard care despite limited evidence. Although it is safe in selected patients at appropriate dosages, magnesium may cause adverse effects or death at high dosages. Because magnesium is excreted renally, it should be used with caution in patients with kidney disease. Food sources of magnesium include green leafy vegetables, nuts, legumes, and whole grains. (Am Fam Physician. 2009;80(2):157-162. Copyright © 2009 American Academy of Family Physicians.) agnesium is the fourth most use (e.g., diuretics, antibiotics); alcoholism; abundant essential mineral and older age (e.g., decreased absorption of in the body. It is distributed magnesium, increased renal exertion).1 approximately one half in the There are challenges in diagnosing mag- Mbone and one half in the muscle and other nesium deficiency because of its distribution soft tissues; less than one percent is in the in the body. -

Obstetric Safety
Obstetric Safety DIAGNOSIS OF LABOUR FIRST STAGE . Latent phase . Cervix less than 4 cm dilated . Active phase . Cervix between 4 cm and 10 cm dilated SECOND STAGE . Early phase (non-expulsive) . Cervix fully dilated (10 cm) . Fetal descent continues . Patient has no urge to push . Late phase (expulsive) . Presenting part of fetus reaches the pelvic floor and the patient has the urge to push . Typically lasts less than 1 hour in primigravida women and less than 30 minutes in multigravida women Carry .out Dedicated vaginal examinations equipment at leastfor procedures once every 4 hours in the first stage of labour. andEquipment plot the findings to monitor on the patients, partograph. as required for the procedure . Drugs and other consumables for routine and emergency use The partograph is very helpful in monitoring the progress of labour and in the early detection of abnormal labour patterns. WHO/HPW/CPR 2004 reformatted 2012 1 DIAGNOSIS OF VAGINAL BLEEDING IN EARLY PREGNANCY Typical Symptoms and Probable Occasional Symptoms and Signs Signs Diagnosis . Light* bleeding . Cramping/lower abdominal pain Threatened . Closed cervix . Uterus softer than normal abortion . Uterus corresponds to dates . Light bleeding . Abdominal pain . Fainting . Closed cervix . Tender adnexal mass Ectopic . Uterus slightly larger than . Amenorrhea pregnancy normal . Cervical motion tenderness . Uterus softer than normal . Light bleeding . Light cramping/lower abdominal pain . Closed cervix Complete . History of expulsion of products of . Uterus smaller than dates abortion conception . Uterus softer than normal . Cramping/lower abdominal pain . Heavy** bleeding . Tender uterus Inevitable . Dilated cervix . No expulsion of products of abortion . Uterus corresponds to dates conception . Heavy bleeding . -

The Regional Center for Poison Control and Prevention Serving Massachusetts and Rhode Island
The Regional Center for Poison Control and Prevention Serving Massachusetts and Rhode Island Poison Potential Antidote Acetaminophen n-Acetylcysteine [Mucomyst®] Anticholinergics Physostigmine [Antilirium®] Benzodiazepines Flumazenil [Romazicon®] Beta-adrenergic blockers Glucagon Botulinum toxin Trivalent ABE botulinum antitoxin Calcium chloride or calcium gluconate Calcium channel blockers Hyperinsulinemia-euglycemia (HIE) therapy Atropine Carbamates Pralidoxime (2-PAM) [Protopam®] Carbon monoxide Oxygen; Hyperbaric oxygen (HBO) Clonidine Naloxone [Narcan®] Cyanide Cyanide Kit (Amyl/sodium nitrite, sodium thiosulfate) Digoxin (Cardiac glycosides) Digoxin Immune FAB Ovine [Digibind®, Digifab®] Epi Pen (Epinephrine SQ) Phentolamine [Regitine®] Ethanol Ethylene glycol 4-Methylpyrazole (Fomepizole) [Antizol®] Fluoride Calcium chloride or calcium gluconate Heparins Protamine Hydrofluoric acid Calcium chloride or calcium gluconate Hydrogen sulfide Oxygen; Hyperbaric oxygen (HBO); Sodium nitrite Iodine Starch Isoniazid Pyridoxine (Vitamin B6 ) METALS Dimercaprol [BAL] Arsenic Dimercaptosuccinic acid (DMSA, succimer) [Chemet®] Bismuth Dimercaprol [BAL] Copper D-Penicillamine [Cuprimine®] Gold Dimercaprol [BAL] Iron Deferoxamine [Desferal®] Dimercaprol [BAL] Edetate calcium disodium (Calcium EDTA) Lead Dimercaptosuccinic acid (DMSA, succimer) [Chemet®] D-Penicillamine [Cuprimine®] Dimercaprol [BAL] Mercury Dimercaptosuccinic acid (DMSA, succimer) [Chemet®] Ethanol Methanol 4-Methylpyrazole (Fomepizole) [Antizol®] Methemoglobinemic agents Methylene