Wednesday, February 10, 2016 4 P.M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
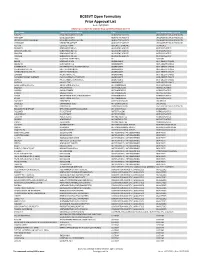
BCBSVT Open Formulary Prior Approval List
BCBSVT Open Formulary Prior Approval List As of: 10/27/2020 Helpful Tip: To search for a specific drug, use the find feature (Ctrl + F) Trade Name Chemical/Biological Name Class Prior Authorization Program FUSILEV LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS KHAPZORY LEVOLEUCOVORIN ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS LEVOLEUCOVORIN CALCIUM LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS VISTOGARD URIDINE TRIACETATE ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS ACTHAR CORTICOTROPIN ADRENAL HORMONES HORMONES BELRAPZO BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDAMUSTINE HCL BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDEKA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS TREANDA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS DAW (DISPENSE AS WRITTEN) ALL CUSTOM BELVIQ LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS BELVIQ XR LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS CONTRAVE ER NALTREXONE HCL/BUPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL ER DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS LOMAIRA PHENTERMINE HCL ANOREXIANTS ANTI‐OBESITY DRUGS PHENDIMETRAZINE TARTRATE PHENDIMETRAZINE TARTRATE ANOREXIANTS ANTI‐OBESITY DRUGS QSYMIA PHENTERMINE/TOPIRAMATE ANOREXIANTS ANTI‐OBESITY DRUGS SAXENDA LIRAGLUTIDE ANOREXIANTS ANTI‐OBESITY DRUGS ABIRATERONE ACETATE ABIRATERONE ACETATE ANTIANDROGENS ANTINEOPLASTICS ERLEADA APALUTAMIDE ANTIANDROGENS ANTINEOPLASTICS NUBEQA DAROLUTAMIDE ANTIANDROGENS -

What Are the Acute Treatments for Migraine and How Are They Used?
2. Acute Treatment CQ II-2-1 What are the acute treatments for migraine and how are they used? Recommendation The mainstay of acute treatment for migraine is pharmacotherapy. The drugs used include (1) acetaminophen, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans and (5) antiemetics. Stratified treatment according to the severity of migraine is recommended: use NSAIDs such as aspirin and naproxen for mild to moderate headache, and use triptans for moderate to severe headache, or even mild to moderate headache when NSAIDs were ineffective in the past. It is necessary to give guidance and cautions to patients having acute attacks, and explain the methods of using medications (timing, dose, frequency of use) and medication use during pregnancy and breast-feeding. Grade A Background and Objective The objective of acute treatment is to resolve the migraine attack completely and rapidly and restore the patient’s normal functions. An ideal treatment should have the following characteristics: (1) resolves pain and associated symptoms rapidly; (2) is consistently effective; (3) no recurrence; (4) no need for additional use of medication; (5) no adverse effects; (6) can be administered by the patients themselves; and (7) low cost. Literature was searched to identify acute treatments that satisfy the above conditions. Comments and Evidence The acute treatment drugs for migraine generally include (1) acetaminophens, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans, and (5) antiemetics. For severe migraines including status migrainosus and migraine attacks refractory to treatment, (6) anesthetics, and (7) corticosteroids (dexamethasone) are used (Tables 1 and 2).1)-9) There are two approaches to the selection and sequencing of these medications: “step care” and “stratified care”. -

Novel Formulations for Treatment of Migraine
(19) TZZ _T (11) EP 2 756 756 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 23.07.2014 Bulletin 2014/30 A01N 43/42 (2006.01) A61K 31/44 (2006.01) (21) Application number: 14165112.5 (22) Date of filing: 24.04.2009 (84) Designated Contracting States: • Turanin, John AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Emeryville, CA California 94608 (US) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL • Hawley, Roger PT RO SE SI SK TR Emeryville, CA California 94608 (US) • Schuster, Jeffrey, A. (30) Priority: 28.04.2008 US 48463 Bolinas, CA California 94924 (US) (62) Document number(s) of the earlier application(s) in (74) Representative: Duxbury, Stephen et al accordance with Art. 76 EPC: Arnold & Siedsma 09739139.5 / 2 273 880 Pettenkoferstrasse 37 80336 München (DE) (71) Applicant: Zogenix, Inc. Emeryville CA 94608 (US) Remarks: This application was filed on 17-04-2014 as a (72) Inventors: divisional application to the application mentioned • Farr, Stephen J. under INID code 62. Orinda, CA California 94563 (US) (54) Novel formulations for treatment of migraine (57) Systems and methods are described for treating Systems that are self contained, portable, prefilled, and un-met medical needs in migraine and related conditions simple to self administer at the onset of a migraine attack such as cluster headache. Included are treatments that are disclosed, and preferably include a needle-free in- are both rapid onset and long acting, which include sus- jector and a high viscosity formulation, to eliminate such tained release formulations, and combination products, issues as fear of self administration with needles, and Also included are treatments for multiple symptoms of needle stick and cross contamination. -

2018-2019 Targeted Medication Safety Best Practices for Hospitals
2018-2019 Targeted Medication Safety Best Practices for Hospitals The purpose of the Targeted Medication Safety Best Practices for Hospitals is to identify, inspire, and mobilize widespread, national adoption of consensus-based best practices for specific medication safety issues that continue to cause fatal and harmful errors in patients, despite repeated warnings in ISMP publications. Hospitals can focus their medication safety efforts over the next 2 years on these best practices, which are realistic and have been successfully adopted by numerous organizations. While targeted for the hospital-based setting, some best practices may be applicable to other healthcare settings. The Targeted Medication Safety Best Practices for Hospitals have been reviewed by an external expert advisory panel and approved by the ISMP Board of Trustees. Related issues of the ISMP Medication Safety Alert! are referenced after each best practice. ISMP encourages hospitals that have not implemented the 2016-2017 Targeted Medication Safety Best Practices for Hospitals to do so as a priority, while implementing the 2018-2019 best practices. Organizations need to focus on previous best practices 2, 3, 9 and 11 since these have the lowest implementation rate. Two of the 2016-2017 Targeted Medication Safety Best Practices for Hospitals (number 4 and 7) have been revised for 2018-2019. Best practices number 12 through 14 are new for 2018-2019. www.ismp.org BEST PRACTICE 1: Dispense vinCRIStine (and other vinca alkaloids) in a minibag of a compatible solution and not in a syringe. Rationale: Related ISMP Medication The goal of this best practice is to ensure that vinca alkaloids are Safety Alerts!: administered by the intravenous route only. -

(12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness Et Al
USOO6264,917B1 (12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness et al. (45) Date of Patent: Jul. 24, 2001 (54) TARGETED ULTRASOUND CONTRAST 5,733,572 3/1998 Unger et al.. AGENTS 5,780,010 7/1998 Lanza et al. 5,846,517 12/1998 Unger .................................. 424/9.52 (75) Inventors: Jo Klaveness; Pál Rongved; Dagfinn 5,849,727 12/1998 Porter et al. ......................... 514/156 Lovhaug, all of Oslo (NO) 5,910,300 6/1999 Tournier et al. .................... 424/9.34 FOREIGN PATENT DOCUMENTS (73) Assignee: Nycomed Imaging AS, Oslo (NO) 2 145 SOS 4/1994 (CA). (*) Notice: Subject to any disclaimer, the term of this 19 626 530 1/1998 (DE). patent is extended or adjusted under 35 O 727 225 8/1996 (EP). U.S.C. 154(b) by 0 days. WO91/15244 10/1991 (WO). WO 93/20802 10/1993 (WO). WO 94/07539 4/1994 (WO). (21) Appl. No.: 08/958,993 WO 94/28873 12/1994 (WO). WO 94/28874 12/1994 (WO). (22) Filed: Oct. 28, 1997 WO95/03356 2/1995 (WO). WO95/03357 2/1995 (WO). Related U.S. Application Data WO95/07072 3/1995 (WO). (60) Provisional application No. 60/049.264, filed on Jun. 7, WO95/15118 6/1995 (WO). 1997, provisional application No. 60/049,265, filed on Jun. WO 96/39149 12/1996 (WO). 7, 1997, and provisional application No. 60/049.268, filed WO 96/40277 12/1996 (WO). on Jun. 7, 1997. WO 96/40285 12/1996 (WO). (30) Foreign Application Priority Data WO 96/41647 12/1996 (WO). -

Contractile Responses to Ergotamine and Dihydroergotamine in The
J Headache Pain (2007) 8:83-89 DOI 10.1007/s10194-007-0368-9 ORIGINAL Peer Tfelt-Hansen Contractile responses to ergotamine and Elisabeth Nilsson Lars Edvinsson dihydroergotamine in the perfused middle cerebral artery of rat Received: 2 November 2006 Abstract The vasomotor effects of 7.6±0.2 for ergotamine and Accepted in revised form: 22 January 2007 ergotamine and dihydroergotamine 8.4±0.5 for DHE. The responses Published online: 11 May 2007 (DHE) on the middle cerebral were blocked by the 5-HT2A recep- artery (MCA) of rats were studied tor antagonist ketanserin (concen- using the pressurised arteriography tration 10–12 to 10–5 M) and partial- method and in vitro myographs. ly with the 5-HT1B receptor antago- MCAs from Sprague–Dawley rats nist BRL-11557PM-B. The 5-HT1D were mounted on two glass receptor antagonist SB-224289-A micropipettes using the arterio- had no significant effect. Using a graph, pressurised to 85 mmHg myograph technique, isolated ring and luminally perfused. All vessels segments of the MCA with intact used attained spontaneous contrac- endothelium were mounted on two tile tone (34.9±1.8% of resting metal wires. Neither agonist P. Tfelt-Hansen tone) and responded to luminal caused relaxation of resting ves- Department of Neurology, adenosine triphosphate (ATP) with sels, however, they both responded Copenhagen University Hospital, dilatation (24.1±4.0%), which by weak contractile responses Glostrup, Denmark showed functioning endothelium. (26±3% of submaximal contractile E. Nilsson (౧) • L. Edvinsson Luminally added ergotamine or capacity relative to 60 mM potassi- Department of Internal Medicine, DHE induced maximal contrac- um). -

Acute Reversible Cerebral Vasoconstriction Syndrome with Low-Dose Dihydroergotamine Possibly Potentiated by Valproic Acid and Erenumab: a Case Report
Case Report J Neurol Res. 2020;10(1):20-24 Acute Reversible Cerebral Vasoconstriction Syndrome With Low-Dose Dihydroergotamine Possibly Potentiated by Valproic Acid and Erenumab: A Case Report Hsiangkuo Yuana, Stephanie J. Nahasa, Matthew A. Berka, b Abstract disabling headache attacks believed to be due to trigeminovas- cular dysfunction, can be challenging. Migraine preventives Dihydroergotamine (DHE), in the setting of polypharmacy, may increase carried over from medications designed for other medical con- the possibility of reversible cerebral vasoconstriction syndrome (RCVS). ditions (e.g., antiepileptics, antihypertensives, antidepressants) A 64-year-old woman with chronic migraine and medication-overuse commonly display unique adverse event (AE) profiles that headache (on amitriptyline, duloxetine, erenumab) was electively ad- can lead to reduced medication adherence and poor migraine mitted for 5 days of intravenous (IV) ketamine (up to 55 mg/h) to treat control [1]. In refractory migraine, use of intravenous (IV) intractable migraine pain. On the seventh day, upon receiving her forth medications such as lidocaine, dihydroergotamine (DHE), dose of IV DHE (0.25 mg) and the first of IV valproic acid (VPA) (500 or ketamine may offer acute relief [2, 3]. Despite achieving mg) adjunctively, she developed acute bilateral decreased visual acuity, greater headache control by using a cocktail of medications, bitemporal visual field deficit, and unsteady gait. Brain magnetic reso- unexpected AEs may occur, especially in the setting of poly- nance imaging/magnetic resonance angiography (MRI/MRA) showed pharmacy. Here, we report a case of a woman who developed confluent bilateral T2 hyperintensities with punctate restricted diffusion posterior reversible encephalopathy syndrome (PRES) and in the occipital lobes associated with multi-segmental narrowing involv- reversible cerebral vasoconstriction syndrome (RCVS) while ing anterior, middle, posterior cerebral, and basilar arteries consistent being treated with IV ketamine, neuroleptics, DHE, and other with RCVS. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Database for Drug Metabolism and Comparisons, Nicedrug.Ch, Aids
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.28.120782; this version posted June 30, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Database for drug metabolism and comparisons, NICEdrug.ch, aids discovery and design 2 3 Authors/affiliation 1 1,2,5 1,3,5 4 Homa MohammadiPeyhani , Anush Chiappino-Pepe , Kiandokht Haddadi , Jasmin 1 1,4 1,* 5 Hafner , Noushin Hadadi , Vassily Hatzimanikatis 6 1 7 Laboratory of Computational Systems Biotechnology, École Polytechnique Fédérale de 8 Lausanne, EPFL, Lausanne, Switzerland 2 9 Present address: Department of Genetics, Harvard Medical School, Boston, Massachusetts, 10 USA 3 11 Present address: Department of Chemical Engineering and Applied Chemistry, University of 12 Toronto, Toronto, Canada 4 13 Present address: Department of Cell Physiology and Metabolism, Université de Genève, 14 Geneva, Switzerland 5 15 These authors contributed equally 16 * 17 Corresponding author: 18 Prof. Vassily Hatzimanikatis 19 Laboratory of Computational Systems Biotechnology (LCSB), École Polytechnique Fédérale de 20 Lausanne (EPFL), CH‐1015 21 Lausanne, Switzerland 22 Email: [email protected] , Phone: +41 (0)21 693 98 70, Fax: +41 (0)21 693 98 75 23 24 Abstract 25 The discovery of a drug requires over a decade of enormous research and financial 26 investments—and still has a high risk of failure. To reduce this burden, we developed the 27 NICEdrug.ch database, which incorporates 250,000 bio-active molecules, and studied their 28 metabolic targets, fate, and toxicity. -

New Drug Approvals and Extended Indications for Infants, Children, and Adolescents Marcia L
PEDIATRIC PHARMACOTHERAPY Volume 21 Number 11 November 2015 New Drug Approvals and Extended Indications for Infants, Children, and Adolescents Marcia L. Buck, PharmD, FCCP, FPPAG ver the past six months, a number of which slowly releases the drug over time, O significant new drugs have been approved providing up to 13 hours of symptom control. by the Food and Drug Administration (FDA). In The safety and efficacy of the product was addition, several drugs already on the market established in a phase 3 randomized, placebo- have been granted an indication for use in controlled trial in 108 children with ADHD.4 pediatric patients. Following a 5-week open-label dose optimization period, patients were randomized to New Drug Product Approvals treatment (2.5-10 mg) or placebo for a 1-week period. At the end of the week, scores on the Adapalene and Benzoyl Peroxide Swanson, Kotkin, Agler, M-Flynn, and Pelham Epiduo Forte®, a new product containing (SKAMP)-Combined rating scale were adapalene 0.3%, a retinoid, and benzoyl peroxide compared to baseline. The change in scores after 2.5% was approved on July 16, 2015 for the treatment demonstrated a statistically significant treatment of moderate to severe acne in adults improvement throughout the day compared to and children 12 years of age and older.1 The placebo (assessed at 1, 2, 6, 8, 10, 12, and 13 efficacy of the new product was demonstrated in hours post-dose), with a mean change of -8.8 (SE a phase 3 multicenter randomized, double-blind 1.14) in the treated patients and 6.0 (SE 1.19) in trial comparing it to the gel vehicle without the the controls. -

Specialty Pharmacy Program Drug List
Specialty Pharmacy Program Drug List The Specialty Pharmacy Program covers certain drugs commonly referred to as high-cost Specialty Drugs. To receive in- network benefits/coverage for these drugs, these drugs must be dispensed through a select group of contracted specialty pharmacies or your medical provider. Please call the BCBSLA Customer Service number on the back of your member ID card for information about our contracted specialty pharmacies. All specialty drugs listed below are limited to the retail day supply listed in your benefit plan (typically a 30-day supply). As benefits may vary by group and individual plans, the inclusion of a medication on this list does not imply prescription drug coverage. Please refer to your benefit plan for a complete list of benefits, including specific exclusions, limitations and member cost-sharing amounts you are responsible for such as a deductible, copayment and coinsurance. Brand Name Generic Name Drug Classification 8-MOP methoxsalen Psoralen ACTEMRA SC tocilizumab Monoclonal Antibody/Arthritis ACTHAR corticotropin Adrenocortical Insufficiency ACTIMMUNE interferon gamma 1b Interferon ADCIRCA tadalafil Pulmonary Vasodilator ADEMPAS riociguat Pulmonary Vasodilator AFINITOR everolimus Oncology ALECENSA alectinib Oncology ALKERAN (oral) melphalan Oncology ALUNBRIG brigatinib Oncology AMPYRA ER dalfampridine Multiple Sclerosis APTIVUS tipranavir HIV/AIDS APOKYN apomorphine Parkinson's Disease ARCALYST rilonacept Interleukin Blocker/CAPS ATRIPLA efavirenz-emtricitabine-tenofovir HIV/AIDS AUBAGIO -

(12) Patent Application Publication (10) Pub. No.: US 2010/0081663 A1 Cook Et Al
US 20100081663A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0081663 A1 Cook et al. (43) Pub. Date: Apr. 1, 2010 (54) METHOD OF THERAPEUTIC (60) Provisional application No. 60/900,850, filed on Feb. ADMINISTRATION OF DHE TO ENABLE 11, 2007. RAPID RELEF OF MIGRAINE WHILE MINIMIZING SIDE EFFECT PROFILE Publication Classification (75) Inventors: Robert O. Cook, Hillsborough, NJ (51) Int. Cl. (US): Stephen B. Shrewsbury, A61R 3L/4985 (2006.01) Corvallis, OR (US); Nabih N. A6IP 25/06 (2006.01) Ramadan, Lake Forest, IL (US); A6IM I5/00 (2006.01) Thomas A. Armer, Cupertino, CA (US) (52) U.S. Cl. .................................... 514/250; 128/203.15 Correspondence Address: (57) ABSTRACT MORRISON & FOERSTER LLP 755 PAGE MILL RD Pharmaceutical compositions containing dihydroergotamine PALOALTO, CA 94304-1018 (US) (DHE) and methods in which DHE is administered to patients for treatment of migraine without side effects or adverse (73) Assignee: MAP Pharmaceuticals, Inc., effects are disclosed. Methods for rapid treatment of migraine Mountain View, CA (US) with DHE are disclosed comprising: dampening the peak plasma concentration (C) and slightly delaying the peak (21) Appl. No.: 12/548,292 such as to avoid activating the dopaminergic and adrenergic receptors, while achieving sufficient active binding to the (22) Filed: Aug. 26, 2009 serotonin receptors to providerelief from migraine symptoms within a timeframe that permits rapid resolution of migraine Related U.S. Application Data symptoms. Inhaler devices suitable for the methods are dis (63) Continuation of application No. 12/069,667, filed on closed. Kits for practicing the methods of invention are dis Feb.