How to Administer CYANOKIT (Hydroxocobalamin for Injection) 5 G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Significance and Implications of Vitamin B-12 Reaction Shema- ETH ZURICH VARIANT: Mechanisms and Insights
Taylor University Pillars at Taylor University Student Scholarship: Chemistry Chemistry and Biochemistry Fall 2019 Significance and Implications of Vitamin B-12 Reaction Shema- ETH ZURICH VARIANT: Mechanisms and Insights David Joshua Ferguson Follow this and additional works at: https://pillars.taylor.edu/chemistry-student Part of the Analytical Chemistry Commons, Inorganic Chemistry Commons, Organic Chemistry Commons, Other Chemistry Commons, and the Physical Chemistry Commons CHEMISTRY THESIS SIGNIFICANCE AND IMPLICATIONS OF VITAMIN B-12 REACTION SCHEMA- ETH ZURICH VARIANT: MECHANISMS AND INSIGHTS DAVID JOSHUA FERGUSON 2019 2 Table of Contents: Chapter 1 6 Chapter 2 17 Chapter 3 40 Chapter 4 59 Chapter 5 82 Chapter 6 118 Chapter 7 122 Appendix References 3 Chapter 1 A. INTRODUCTION. Vitamin B-12 otherwise known as cyanocobalamin is a compound with synthetic elegance. Considering how it is composed of an aromatic macrocyclic corrin there are key features of this molecule that are observed either in its synthesis of in the biochemical reactions it plays a role in whether they be isomerization reactions or transfer reactions. In this paper the focus for the discussion will be on the history, chemical significance and total synthesis of vitamin B12. Even more so the paper will be concentrated one of the two variants of the vitamin B-12 synthesis, namely the ETH Zurich variant spearheaded by Albert Eschenmoser.Examining the structure as a whole it is observed that a large portion of the vitamin B12 is a corrin structure with a cobalt ion in the center of the macrocyclic part, and that same cobalt ion has cyanide ligands. -

Magnesium-Protoporphyrin Chelatase of Rhodobacter
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 1941-1944, March 1995 Biochemistry Magnesium-protoporphyrin chelatase of Rhodobacter sphaeroides: Reconstitution of activity by combining the products of the bchH, -I, and -D genes expressed in Escherichia coli (protoporphyrin IX/tetrapyrrole/chlorophyll/bacteriochlorophyll/photosynthesis) LUCIEN C. D. GIBSON*, ROBERT D. WILLOWSt, C. GAMINI KANNANGARAt, DITER VON WETTSTEINt, AND C. NEIL HUNTER* *Krebs Institute for Biomolecular Research and Robert Hill Institute for Photosynthesis, Department of Molecular Biology and Biotechnology, University of Sheffield, Sheffield, S10 2TN, United Kingdom; and tCarlsberg Laboratory, Department of Physiology, Gamle Carlsberg Vej 10, DK-2500 Copenhagen Valby, Denmark Contributed by Diter von Wettstein, November 14, 1994 ABSTRACT Magnesium-protoporphyrin chelatase lies at Escherichia coli and demonstrate that the extracts of the E. coli the branch point of the heme and (bacterio)chlorophyll bio- transformants can convert Mg-protoporphyrin IX to Mg- synthetic pathways. In this work, the photosynthetic bacte- protoporphyrin monomethyl ester (20, 21). Apart from posi- rium Rhodobacter sphaeroides has been used as a model system tively identifying bchM as the gene encoding the Mg- for the study of this reaction. The bchH and the bchI and -D protoporphyrin methyltransferase, this work opens up the genes from R. sphaeroides were expressed in Escherichia coli. possibility of extending this approach to other parts of the When cell-free extracts from strains expressing BchH, BchI, pathway. In this paper, we report the expression of the genes and BchD were combined, the mixture was able to catalyze the bchH, -I, and -D from R. sphaeroides in E. coli: extracts from insertion of Mg into protoporphyrin IX in an ATP-dependent these transformants, when combined in vitro, are highly active manner. -

Thiosulfate De Sodium Sterop
Thiosulfate de Sodium Sterop 1. DENOMINATION DU MEDICAMENT THIOSULFATE DE SODIUM STEROP 1g/5ml solution injectable 2. COMPOSITION QUALITATIVE ET QUANTITATIVE Substance active : Une ampoule de 5ml contient 1g de thiosulfate de sodium dans de l’eau pour préparations injectables. 1 ml de solution contient 200mg de thiosulfate de sodium. Pour la liste complète des excipients, voir rubrique 6.1. 3. FORME PHARMACEUTIQUE Solution injectable. 4. DONNEES CLINIQUES 4.1 Indications thérapeutiques - Antidote dans le traitement des intoxications par les cyanures ainsi que par le nitroprussiate de sodium. - Prévention des effets néphrotoxiques du cisplatine. Thiosulfate de Sodium Sterop © Pharma.be Pagina 1 van 6 4.2 Posologie et mode d’administration Mode d'administration Pour voie intraveineuse lente. Posologie Intoxications par les cyanures: Adultes: La posologie habituelle est de 12,5 g de thiosulfate de sodium, administré par voie I.V. sur une période de 5 à 10 minutes. Enfants: La posologie habituelle est de 412,5 mg de thiosulfate de sodium par kg, administré par voie I.V. à la vitesse de 0,625 à 1,25 g/minute sur une période de 5 à 10 minutes. Si les symptômes persistent ou réapparaissent dans la demi-heure ou dans l’heure ayant suivi la première injection, une seconde injection de thiosulfate de sodium est nécessaire avec comme posologie la moitié de celle de la première injection. En prévention des effets néphrotoxiques du cisplatine: Chez l'adulte, la posologie initiale est de 9 g de thiosulfate de sodium par m2 de surface corporelle, administré par voie I.V. Elle est suivie par l'injection de 1,2 g de thiosulfate de sodium par m2 de surface corporelle par heure, en perfusion I.V. -

Adult BLS Standing Orders • Ensure EMS Provider Safety, Consider HAZMAT Activation
Imperial County Public Health Department Emergency Medical Services Agency Policy/Procedure/Protocol Manual Treatment Protocols Date: 07/01/2021 Poisoning/Intoxication/Envenomation - Adult Policy #9160A Adult BLS Standing Orders • Ensure EMS provider safety, consider HAZMAT activation. Recognize, Notify, Isolate • Universal Patient Protocol • Do not approach patient or location if scene safety is in question • Obtain accurate history of incident: o Name of product or substance o Quantity ingested, and/or duration of exposure o Time elapsed since exposure o If safe and accessible, bring medications or bottles to hospital • Move victim(s) to safe environment • Externally decontaminate - PRN • Continuously monitor ECG, blood pressure, pulse oximetry, and capnography (if ALS present) PRN • Give oxygen and provide airway support per Airway Policy • Contact Poison Control Center as needed 1 (800) 222-1222 Suspected Opioid Overdose with Respirations <12 RPM • If possible, avoid the use of a supraglottic device prior to the administration of naloxone • Administer naloxone 0.1 mg/kg, max of 2 mg IN. May repeat up to three (3) times, q5min • May assist family/friends on-scene with administration of patient’s own naloxone • NOTE - Use with caution in opioid dependent pain management patients • Assess vitals, with specific attention to respiratory rate and respiratory drive • Note pupil exam • Note drug paraphernalia or medication bottles near patient Suspected Stimulant Overdose with Sudden Hypoventilation, Oxygen Desaturation, or Apnea • High flow -

Hydrogen Sulfide Metabolite, Sodium Thiosulfate
International Journal of Molecular Sciences Review Hydrogen Sulfide Metabolite, Sodium Thiosulfate: Clinical Applications and Underlying Molecular Mechanisms Max Y. Zhang 1,2, George J. Dugbartey 1,2,3, Smriti Juriasingani 1,3 and Alp Sener 1,2,3,4,* 1 Matthew Mailing Center for Translational Transplant Studies, London Health Sciences Center, Western University, London, ON N6A 5A5, Canada; [email protected] (M.Y.Z.); [email protected] (G.J.D.); [email protected] (S.J.) 2 London Health Sciences Center, Multi-Organ Transplant Program, Western University, London, ON N6A 5A5, Canada 3 London Health Sciences Center, Department of Surgery, Division of Urology, Western University, London, ON N6A 5A5, Canada 4 Department of Microbiology & Immunology, Schulich School of Medicine & Dentistry, University of Western Ontario, London, ON N6A 3K7, Canada * Correspondence: [email protected]; Tel.: +1(519) 6633352 Abstract: Thiosulfate in the form of sodium thiosulfate (STS) is a major oxidation product of hydrogen sulfide (H2S), an endogenous signaling molecule and the third member of the gasotransmitter family. STS is currently used in the clinical treatment of acute cyanide poisoning, cisplatin toxicities in cancer therapy, and calciphylaxis in dialysis patients. Burgeoning evidence show that STS has antioxidant and anti-inflammatory properties, making it a potential therapeutic candidate molecule that can target multiple molecular pathways in various diseases and drug-induced toxicities. This review Citation: Zhang, M.Y.; Dugbartey, discusses the biochemical and molecular pathways in the generation of STS from H2S, its clinical G.J.; Juriasingani, S.; Sener, A. usefulness, and potential clinical applications, as well as the molecular mechanisms underlying these Hydrogen Sulfide Metabolite, clinical applications and a future perspective in kidney transplantation. -

Ingestions, Intoxications, and the Critically Ill Child Poisoning in Children
Ingestions, Intoxications, and the Critically Ill Child Poisoning in Children • 1 million cases of exposure to toxins in children younger than 6 years reported in the U.S. In 1993 • estimated that another 1 million exposures to toxins not reported • 1% have moderate or major life-threatening symptoms • 60-100 deaths annually in the U.S Poisoning in Children Less Than 5 Years Old • accounts for 85-90% of pediatric poisoning • is generally accidental • secondary to exploratory behavior and lack of supervision • tend to involve single agent ingestions Poisoning in Children Over 5 Years Old • accounts for 10-15% of pediatric poisoning • is generally intentional • secondary to suicide attempts or gestures, or to intoxications and inadvertent overdose • tend to involve multiple agent ingestions General Concepts for Pediatric Poisoning • Prevention • Initial Stabilization • Diagnosis • Specific Antidotes Management of the Poisoned Child • Treat the Patient, Not the Poison --patient-specific treatment is safer, less expensive, and more effective Management of the Poisoned Child • Stabilization --Airway --Breathing --Circulation --Disability (neurologic) Management of the Poisoned Child • Respiratory Failure --airway obstruction from secretions, refluxed gastric contents, airway muscle relaxation --respiratory muscle rigidity --loss of respiratory drive --pulmonary edema Management of the Poisoned Child • Cardiovascular Collapse --arteriolar dilation --venous dilation --myocardial depression --dysrhythmias Management of the Poisoned Child • Neurologic -

Antidote List
Antidote List Recommended minimum amounts for hospital pharmacies to stock Call the Maryland Poison Center for expert advice on the treatment of all poisonings and overdoses: 1-800-222-1222 Poisoning Minimum Stocking Antidote Indication Recommendations Oral: 120 grams Acetylcysteine Acetaminophen IV: 96 grams Crotaline snake Antivenin, snake (CroFab®) 12 vials envenomation 1 vial Black widow spider (Note: Merck limits distribution Antivenin, black widow spider envenomation to cases of confirmed bites with symptoms only) Organophosphate & Atropine carbamate insecticides, nerve 165 mg gases Calcium chloride Calcium channel blockers 10 grams 2.25 grams Calcium disodium EDTA Lead (Available direct from ASD Specialty Healthcare) Hydrofluoric acid 1 kg (powder) Calcium gluconate Calcium channel blockers IV: 30 grams 1 kit Cyanide Antidote Kit Cyanide (or Hydroxocobalamin, see below) Cyproheptadine (Periactin®) Serotonin syndrome 80 mg Neuroleptic malignant Dantrolene syndrome, stimulant-induced 720 mg hyperthermia Deferoxamine mesylate Iron 8 grams (Desferal®) Digoxin Immune FAB Digoxin 20 vials (Digibind®, DigiFab®) Dimercaprol (BAL) Heavy metals 1.5 grams 1 | P a g e Maryland Poison Center Antidote List – continued Poisoning Minimum Stocking Antidote Indication Recommendations DMSA (Succimer, Chemet®) Heavy metals 2000 mg Folic acid Methanol IV: 150 mg Flumazenil (Romazicon®) Benzodiazepines 10 mg Fomepizole (Antizol®) Ethylene glycol, methanol 12 grams Beta blockers, Glucagon 50 mg calcium channel blockers Hydroxocobalamin (Cyanokit®) Cyanide -

1 INTRODUCTION Corroles Are Tetrapyrrolic Molecules, Maintaining
1 Chapter 1 INTRODUCTION Corroles are tetrapyrrolic molecules, maintaining the skeletal structure of corrin, with its three meso carbon positions and one direct pyrrole-pyrrole linkage, and possessing the aromaticity of porphyrins (see figure 1.1). First reported by Johnson and Kay in 1965[1], corroles were the end product of a many step synthetic scheme, finally being formed by the photocyclization of a,c-biladienes. While this last step was simple, with 20-60% yields, the route to a,c-biladienes was far from easy. Indeed, the overall reaction from readily available starting materials to corrole was a multi-step synthesis, with poor yields in many of the reactions. Thus, while corroles have been known for more than 40 years, research in the field was slow to progress. It wasn’t until the discovery of new synthetic methods for corroles developed in 1999 by different groups working independently that research in this area really started to expand[2]. This is not to say, however, that the field of corrole research was non-existent prior to the development of the new methodologies. While it was slow growing after the initial publication, it was far from stagnant, and many of the interesting properties of corroles were investigated by different groups. Among these properties is their ability to stabilize unusually high formal oxidation states of metal ions, such as iron(IV), cobalt(IV), and cobalt(V)[3, 4]. In fact, one particular corrole available through a method developed by Zeev 2 N N H N N H H N N N N a b N N H H H N N c Figure 1.1. -

ABSTRACT ZHANG, SHAOFEI. Synthesis Of
ABSTRACT ZHANG, SHAOFEI. Synthesis of Bacteriochlorins and the Full Skeleton of Bacteriochlorophylls. (Under the direction of Professor Jonathan S. Lindsey.) Bacteriochlorins, the core macrocycle ring of natural bacteriochlorophylls, are characterized by their ability to absorb near infrared light (700 – 900 nm), which makes them attractive candidates in variety of photophysical studies and applications. The previously established de novo synthesis, which relies on the acid-catalyzed self-condensation of dihydrodipyrrin–acetal, provides access towards diverse bacteriochlorins, but has its limitations. This dissertation describes the development of new strategies for bacteriochlorin synthesis. Firstly, a route towards previously unknown tetra-alkyl bacteriochlorins (e.g., alkyl = Me, or –CH2CO2Me) is established (Ch. 2). Secondly, explorations of synthetic approaches to unsymmetrically substituted bacteriochlorins through electrocyclic reactions of linear tetrapyrrole intermediates are described. Four new unsymmetrically substituted bacteriochlorins and one new tetradehydrocorrin were produced, albeit in low yields (Ch. 3). Thirdly, a new method to construct bacteriochlorin macrocycle with concomitant Nazarov cyclization to form the annulated isocyclic ring, is established. Five new bacteriochlorins, which are closely anlogues of bacteriochlorophyll a, bearing various substituents (alkyl/alkyl, aryl, and alkyl/ester) at positions 2 and 3 and 132 carboalkoxy groups (R = Me or Et) were constructed in 37−61% yield from the bilin analogues -
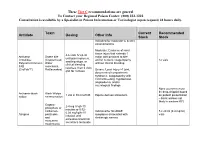
Antidote Stocking Tier C
These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Toxin Current Recommended Antidote Dosing Other Info Stock Stock Indicated for moderate to severe envenomations Moderate: Evidence of local tissue injury that extends 1 4-6 vials IV q2-4h Antivenin Snake bite major joint proximal to bite until pain improves, Crotalidae (Copperhead, and/or numeric coagulopathy 12 vials swelling stops, or Polyvalent Immune Water without clinical bleeding clinical bleeding FAB moccasins, resolves, then 2 vials (CroFab™) Rattlesnakes) Severe: Local injury >1 joint, q6h for 3 doses documented compartment syndrome, coagulopathy with clinical bleeding, hypotension, angioedema, and/or neurological findings None (currently must be drop-shipped based Antivenin-black Black Widow 1 vial in 50 ml of NS Equine derived antivenom on patient presentation widow envenomation – black widows not likely in western KY) Organo- 2-4 mg IV q5-10 phosphate or minutes or 0.02- carbamate Indicated for SLUDGE 5 x 20 ml (0.4 mg/ml) 0.08 mg/kg/hr IV Atropine pesticides symptoms associated with vials infusion until and cholinergic excess excessive bronchial muscarine secretions terminate mushrooms These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Antidote Current Recommended Toxin -

Cheminformatics for Genome-Scale Metabolic Reconstructions
CHEMINFORMATICS FOR GENOME-SCALE METABOLIC RECONSTRUCTIONS John W. May European Molecular Biology Laboratory European Bioinformatics Institute University of Cambridge Homerton College A thesis submitted for the degree of Doctor of Philosophy June 2014 Declaration This thesis is the result of my own work and includes nothing which is the outcome of work done in collaboration except where specifically indicated in the text. This dissertation is not substantially the same as any I have submitted for a degree, diploma or other qualification at any other university, and no part has already been, or is currently being submitted for any degree, diploma or other qualification. This dissertation does not exceed the specified length limit of 60,000 words as defined by the Biology Degree Committee. This dissertation has been typeset using LATEX in 11 pt Palatino, one and half spaced, according to the specifications defined by the Board of Graduate Studies and the Biology Degree Committee. June 2014 John W. May to Róisín Acknowledgements This work was carried out in the Cheminformatics and Metabolism Group at the European Bioinformatics Institute (EMBL-EBI). The project was fund- ed by Unilever, the Biotechnology and Biological Sciences Research Coun- cil [BB/I532153/1], and the European Molecular Biology Laboratory. I would like to thank my supervisor, Christoph Steinbeck for his guidance and providing intellectual freedom. I am also thankful to each member of my thesis advisory committee: Gordon James, Julio Saez-Rodriguez, Kiran Patil, and Gos Micklem who gave their time, advice, and guidance. I am thankful to all members of the Cheminformatics and Metabolism Group. -

A Primitive Pathway of Porphyrin Biosynthesis and Enzymology in Desulfovibrio Vulgaris
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 4853–4858, April 1998 Biochemistry A primitive pathway of porphyrin biosynthesis and enzymology in Desulfovibrio vulgaris TETSUO ISHIDA*, LING YU*, HIDEO AKUTSU†,KIYOSHI OZAWA†,SHOSUKE KAWANISHI‡,AKIRA SETO§, i TOSHIRO INUBUSHI¶, AND SEIYO SANO* Departments of *Biochemistry and §Microbiology and ¶Division of Biophysics, Molecular Neurobiology Research Center, Shiga University of Medical Science, Seta, Ohtsu, Shiga 520-21, Japan; †Department of Bioengineering, Faculty of Engineering, Yokohama National University, 156 Tokiwadai, Hodogaya-ku, Yokohama 240, Japan; and ‡Department of Public Health, Graduate School of Medicine, Kyoto University, Sakyou-ku, Kyoto 606, Japan Communicated by Rudi Schmid, University of California, San Francisco, CA, February 23, 1998 (received for review March 15, 1998) ABSTRACT Culture of Desulfovibrio vulgaris in a medium billion years ago (3). Therefore, it is important to establish the supplemented with 5-aminolevulinic acid and L-methionine- biosynthetic pathway of porphyrins in D. vulgaris, not only methyl-d3 resulted in the formation of porphyrins (sirohydro- from the biochemical point of view, but also from the view- chlorin, coproporphyrin III, and protoporphyrin IX) in which point of molecular evolution. In this paper, we describe a the methyl groups at the C-2 and C-7 positions were deuter- sequence of intermediates in the conversion of uroporphy- ated. A previously unknown hexacarboxylic acid was also rinogen III to coproporphyrinogen III and their stepwise isolated, and its structure was determined to be 12,18- enzymic conversion. didecarboxysirohydrochlorin by mass spectrometry and 1H NMR. These results indicate a primitive pathway of heme biosynthesis in D. vulgaris consisting of the following enzy- MATERIALS AND METHODS matic steps: (i) methylation of the C-2 and C-7 positions of Materials.