Estrum Nocturnum Chandra Kishore Tyagi1*, Deenanath Jhade1, Sunil Kumar Shah1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The First Report of Root-Knot Nematode on Cestrum Nocturnum in Ninawa, Iraq
Plant Archives Volume 20 No. 2, 2020 pp. 6778-6780 e-ISSN:2581-6063 (online), ISSN:0972-5210 THE FIRST REPORT OF ROOT-KNOT NEMATODE ON CESTRUM NOCTURNUM IN NINAWA, IRAQ Firas K. Aljuboori1* and Asmaa Mansour Al-Hakeem2 1*Department of Plant Protection, College of Agriculture and Forestry, University of Mosul, Iraq. 2Department of Microbiology, College of Science, Al-Karkh University of Science, Iraq Abstract Night-blooming jasmine Cestrum nocturnum is one of the common ornamental plants in Iraq. It is widely planted in the house gardens, public gardens and nurseries. A survey was carried out during 2019 to assist the host range of Meloidogyne spp. in ornamental plants. Samples were collected from house gardens, public gardens and nurseries in Ninawa governorate - Iraq. The root-knot nematode was identified by the root symptoms and the presence of females and egg mass. Nematode species was identified depending on the adult female perineal pattern. The most common symptoms of the infected plants were stunting, foliage yellowing, lack of vigor and wilt during the hot summer. Nematode infection was indexed depending on the root galling using a 0-5 scale. Root-knot density and severity were estimated according to both natural and artificial infection. House and public gardens have a higher infection percentage than nurseries (10.4, 1.9) while the severity is higher in nurseries (0.142, 0.35). According to the best of the researchers’ knowledge, this is the first report of Meloidogyne javanica on night-blooming jasmine in Iraq. Key words: root-knot nematode, Cestrum nocturnum, Meloidogyne javanica. Introduction ornamental plants and herbs (Ralmi et al., 2016). -

Delicate, Fragrant, Lady of the Night- a Medicinal Received: 04-09-2016 Accepted: 10-10-2016 Gift
Journal of Medicinal Plants Studies 2016; 4(6): 13-17 ISSN 2320-3862 JMPS 2016; 4(6): 13-17 © 2016 JMPS Delicate, fragrant, lady of the night- A medicinal Received: 04-09-2016 Accepted: 10-10-2016 gift Amin Shaista Department of Pharmaceutical Amin Shaista and Parle Amrita Chemistry, Delhi Pharmaceutical Science and Research University, Abstract New Delhi India Night blooming jasmine, botanically known as Cestrum nocturnum is an evergreen shrub that grows in Parle Amrita tropical and sub-tropical regions throughout the world. Cestrum nocturnum is a popular ornamental plant Department of Pharmaceutical due to its showy and fragrant white flowers. It is also used as a hedge plant and cultivated as a medicinal Chemistry, Delhi Pharmaceutical plant. The medicinal properties of night blooming jasmine include antioxidant, anti-hyperlipidemic, Science and Research University, hepatoprotective, analgesic, antibacterial, antifungal, anti-convulsant, anti-HIV and larvicidal activities. New Delhi India The present paper reviews the geographical distribution, history, cultivation, uses, side effects, synonyms, botanical description, taxonomical classification, phytochemical constituents and pharmacological activities. Keywords: Cestrum nocturnum, antibacterial, antioxidant, anti-inflammatory, larvicidal Introduction Cestrum nocturnum is a garden shrub from the family Solanaceae, commonly known as "lady of the night" which is used as a remedy for different health disorders. This sprawling shrub has glossy simple leaves, vine like stems, greenish-creamy white tubular flowers and fleshy berries. The berries are marfil white or aubergine in colour. The species name ‘nocturnum’ refers to the species’ habit of opening its small, heavily-scented flowers at night. The flowers release powerful sweet perfume at night. It is made into a rare attar (raat ki rani) which is used in Indian and Middle East perfumery. -
CPCB) Guidelines Which Will Help to Attenuate the Pollution Level of Air and Noise
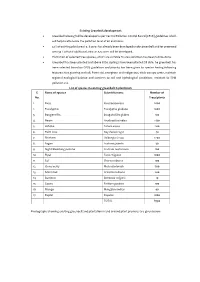
Existing Greenbelt development Greenbelt is being/will be developed as per Central Pollution Control Board (CPCB) guidelines which will help to attenuate the pollution level of air and noise. 33 % of existing plant area i.e. 8 acres has already been developed under greenbelt and for proposed area 33 % of total additional area i.e. 5.5 acres will be developed. Plantation of selected tree species, which are suitable to area condition has been/will be done. Greenbelt has been planted and above 8750 saplings have been planted till date. he greenbelt has been selected based on CPCB guidelines and priority has been given to species having following features; fast growing and tall, Perennial, evergreen and indigenous, thick canopy cover, maintain regional ecological balance and conform to soil and hydrological conditions, resistant to SPM pollution etc. List of species in existing greenbelt & plantation S. Name of species Scientific name Number of No. Trees/plants 1. Ficus Ficus benjamina 2000 2. Eucalyptus Eucalyptus globulus 1000 3. Bougenvillia Bougainvillea glabra 100 4. Neem Azadirachta indica 1130 5. Ashoka Saraca asoca 200 6. Palm Tree Roystonea regia 40 7. Shisham Dalbergia sissoo 1200 8. Sagon Tectona grandis 30 9. Night blooming jasmine Cestrum nocturnum 100 10. Pipal Ficus religiosa 1000 11. Sal Shorea robusta 100 12. China berry Melia azedarach 500 13. Silver Oak Grevillea robusta 200 14. Bamboo Bambusa vulgaris 10 15. Guava Psidium guajava 100 16. Mango Mangifera indica 40 17. Poplar Populus 1000 TOTAL 8750 Photographs showing existing greenbelt and plantation in and around plant premises are given below: Action plan for greenbelt development for proposed area The rate of pollutant removal is found to increase linearly as the concentration of the pollutant increases over the range of concentration that are encountered in ambient air and which are low enough not to cause stomatal closure. -

Cestrum Nocturnum Global Invasive
FULL ACCOUNT FOR: Cestrum nocturnum Cestrum nocturnum System: Terrestrial Kingdom Phylum Class Order Family Plantae Magnoliophyta Magnoliopsida Solanales Solanaceae Common name onaona Iapana (Hawaiian), ike he po (Niuean), thauthau (Fijian), ali'I o le po (Samoan), teine 'o le po (Samoan), galan de noche (Spanish), ye xiang shu (Mandarin), laukau po'uli (Tongan), fafine o te po (Tuvaluan), ala aumoe (Hawaiian), kupaoa (Hawaiian), thauthau ni mbongi (Fijian), tiare ariki va'ine (Cook Islands), jonoul ruo awa (Marshallese), ai pua e pogi (Rotuman), ariki-va'ine (Cook Islands), night jessamine (English), kara (Fijian), dama de noche (Chamorro), night queen (English), lady of the night (English), night cestrum (English), night-blooming jasmine (English), night-flowering cestrum (English), dama di noche (Chamorro), night-flowering jasmine (English), queen of the night (English) Synonym Cestrum parqui Similar species Cestrum parqui Summary Cestrum nocturnum commonly known as queen of the night, is a popular ornamental species widely distributed for its strongly fragrant flowers that bloom at night. Having bird-dispersed seeds and the ability to reproduce vegetatively has resulted in escapes from cultivation, where in some areas it aggressively colonises disturbed sites such as road edges and forest gaps forming dense impenetrable thickets and resulting in competition with and displacement of native plant species. C. nocturnum is also known to be poisonous if ingested, forming a risk to grazing livestock and has been known to produce hay-fever like symptoms in some people. view this species on IUCN Red List Global Invasive Species Database (GISD) 2021. Species profile Cestrum nocturnum. Pag. 1 Available from: http://www.iucngisd.org/gisd/species.php?sc=851 [Accessed 07 October 2021] FULL ACCOUNT FOR: Cestrum nocturnum Species Description Cestrum nocturnum is a glabrous shrub that grows from 1 to 5 m tall (depending on location) with ovate-oblong, petiolate, and obtuse leaves mostly 7 - 20 cm long (Webb et al., 1988; Tharman et al., 1994; Zhang et al., 1994). -

Khin-Lay-Nwe-1.Pdf
3rd Myanmar Korea Conference Research Journal Volume 3, No. 1 211 Comparison on Epidermal Characters of Leaves from Some Members of Family Solanaceae Khin Lay Nwe1, Moe Zin Zin Thet2 Abstract The investigation on the epidermal features of leaves from six species belonging to the family Solanaceae has been undertaken from Hpa-an township, Kayin State. The collected specimens were Capsicum annuum L., Cestrum nocturnum L., Datura fastuosa var. alba (Nees.) Clarke., Lycopersicon esculentum Mill., Physalis micrantha Link, Enum. and Solanum violaceum Ortega, Hort. respectively. The foliar epidermal peels were taken from the both surfaces of leaves. All the investigated species showed anomocytic types of stomata which were abundantly found. The similarities and differences were observed in the cell shape and anticlinal walls of the species. Differences were also found in trichome types. These microscopical characteristics were useful in identification of the species of family Solanaceae. Keywords: epidermal features, leaves, Solanaceae, similarities and differences Introduction The Solanaceae family distributed primarily in tropical America and South America (Lawrence, 1951). This family is occurring throughout the world (Dassanayake, 1987). Solanaceae is a very important economic family (Dassanayake, 1987 and Mukherjee, 2000) .Most members of the family are poisonous due to the presence of tropane or steroid alkaloids. Solanaceae are the source of several pharmaceutical drugs, and some are powerful narcotics (Judd et al., 2002). Kundu and Gupta (1964) stated that the use of microscopy of leaves in distinguishing taxa in Solanaceae. In the present study, epidermal characteristics of the leaves of the six species belonging to the family Solanaceae were studied, described and compared. -

Trichomes Diversity in Family Solanaceae from Muzaffarabad Division, Azad Jammu and Kashmir, Pakistan
INT. J. BIOL. BIOTECH., 15 (1): 99-105, 2018. TRICHOMES DIVERSITY IN FAMILY SOLANACEAE FROM MUZAFFARABAD DIVISION, AZAD JAMMU AND KASHMIR, PAKISTAN Ashfaq Ahmed Awan and Ghulam Murtaza* Department of Botany, University of Azad Jammu and Kashmir, Muzaffarabad. *Corresponding author email:[email protected] ABSTRACT Trichomes of twenty two taxa of the genus Solanum, Withania, Petunia, Capsicum, Atropa, Lycopersicon, Cestrum, Hyoscyamus, Datura, Physalis and Nicotiana were examined by using light microscope and photomicroscope. The indumentums shows considerable variations among different species, and therefore, useable characters in de-limitation of species. The study revealed the characters of anatomic interest of leaf and stem epidermal features that have not been previously reported before and has been conducted first time. Size, shape and types of trichomes were measured with considerable variability and the presence of glandular and non-glandular trichomes were observed. Distribution of foliar trichomes emerged as a supportive taxonomic and anatomic tool, which are present in the species mostly are unicellular, bicellular and non-glandular, glandular with bulbous base. Keywords: Epidermis, anatomy, Trichomes; glandular and non –glandular INTRODUCTION Solanaceae is an important family for possessing countless ornamental, medicinal and nutritious species (Heywood, 1993).The family is represented by about 94 genera and 3000 species of nearly cosmopolitan distribution (Mabberley, 1987) mostly found in tropical and temperate regions with -

Antimicrobial Activities of the Whole Plant of Cestrum Nocturnum Against Pathogenic Microorganisms
African Journal of Microbiology Research Vol. 5(6) pp. 612-616, 18 March, 2011 Available online http://www.academicjournals.org/ajmr DOI: 10.5897/AJMR10.425 ISSN 1996-0808 ©2011 Academic Journals Full Length Research Paper Antimicrobial activities of the whole plant of Cestrum nocturnum against pathogenic microorganisms Murad Ali Khan1*, Humaira Inayat2,3, Haroon Khan4,5, Mohammmad Saeed4, Ikhtair Khan3 and Inayat-Ur-Rahman2 1Department of Chemistry, Kohat University of Science and Technology, Kohat 26000, Pakistan. 2Pakistan Council of Scientific and Industrial Research Laboratories Complex, Jamrud Road, Peshawar, Pakistan. 3Department of Chemistry University of Peshawar, Peshawar 25120, Pakistan. 4Department of Pharmacy University of Peshawar, Peshawar 25120, Pakistan. 5Gandhara College o f Pharmacy, Gandhara University, Peshawar, Pakistan. Accepted 1st March, 2011 The crude methanol extract of the whole plant of Cestrum nocturnum L. (Solanaceae) and its subsequent fractions were tested against various bacterial and fungal strains. With the exception of Salmonella typhi, the tested samples showed marked antibacterial activity against Pseudomonas aeruginosa, Staphylococcus aurous, Bacillus subtilis, Escherichia coli and Shigella flexenari. The minimum inhibitory concentrations (MICs) ranged from 19 to 280 µg/ml. The crude extract and fractions were also susceptible to Candida species and Microsporium canis. The minimum inhibitory concentrations (MICs) for various fungi ranged from 170 to 290 µg/ml. In phytochemical analysis, the crude form and fractions of plant showed the presence of various phytochemical chemical groups like glycosides, alkaloids, saponins, phenols, flavonoids, sterols and tannins. Therefore, the current findings can be attributed to these groups. Key words: Cestrum nocturnum L., antibacterial, antifungal, phytochemicals. INTRODUCTION The genus Cestrum contains more than 300 species, and Zeng et al., 2003; Zeng et al., 2003). -

August – September
August/September2006 Volume 5, Issue 4 M. Bloomers A Garden Journal “Flowers are love’s truest language”…Park Benjamin Fragrance in Full Bloom...Gay Houston, Staff Jasmine — an evocative word...although spring and has finer leaves than the Confeder- late summer and very early fall seem a ate jasmine. All can be planted in the fall for strange time to think of the magical fragrance spring blooms. They are usually evergreen in that the very word jasmine brings to mind. our area and need at least morning sun. Well Now is a great time to plan for next spring's drained, rich soil will bring about better blos- blooms. At Martha's when a blooming plant soms. from this large and varied genus is delivered, Night Blooming Jasmine, Cestrum nocturnum, we place it by the door. Its sweet fragrance is another jasmine that does well and is very entices you to add jasmine to your own gar- vigorous. Since it blooms at night, plant it in den. an area that you can enjoy during the evening This large group of wonderful plants can be hours. Sit back and watch the magic of night somewhat confusing, but in general they usu- feeding moths as they are drawn to the plants ally bloom with white to creamy blossoms, heavy fragrance. have shiny dark green leaves, smell some- Another great plant that is not a true Jasmine what like a gardenia, and thrive in our South- but certainly looks and smells like one is Car- ern climate. That said, we need to say that nation of India, sometimes called Crape Jas- some bloom pink, some yellow, some only at mine. -

Phcog Mag.: Review Article Research and Medicinal Potential of The
Pharmacognosy Reviews Vol 1, Issue 2, Jul-Dec, 2007 PHCOG REV. An official Publication of Phcog.Net Phcog Mag.: Review Article Research and Medicinal Potential of the genus Cestrum (Solanaceae) – A Review A. Sajeli Begum* and Madhur Goyal Department of Pharmaceutics, Institute of Technology, anaras Hindu University, Varanasi-221005, India. Author for correspondence*E-mail: [email protected] ABSTRACT The genus Cestrum belonging to Solanaceae family comprises about 300 species. Cestrum , which was mainly distributed in South America ranges from Southern Florida and Northern Mexico to Chile is now distributed all over the world as an ornamental plant. The genus is rich in saponins and most of the species exhibit toxicity supporting their use as potential insecticide, herbicide, molluscicide, antimicrobial agent and anticancer agent. A characteristic species of this genus, Cestrum diurnum is known for its calcinogenic potential, which can be used as a source of vitamin D in poultry. The present review explores the distribution, chemical composition, pharmacological activity and traditional uses of different species of genus Cestrum. KEY WORDS : Cestrum , Solanaceae, Cestrum diurnum , calcinogenesis, vitamin D 3, saponins. INTRODUCTION Plants are considered to be promising source of medicine in used in Chilean folk medicine as antifebrile and for the the traditional health care system. The efficacy and safety of treatment of fever and inflammation (4). Chinese people use herbal medicine have turned the major pharmaceutical leaves of C. nocturnum for their pharmacological significance population towards medicinal plant’s research. In view of the in burns and swellings. It is also used for treating epilepsy and widespread interest on Solanaceous plants, this work reviews as stupefying charm medicine in West Indian Islands. -

Checklist of the Vascular Plants of San Diego County 5Th Edition
cHeckliSt of tHe vaScUlaR PlaNtS of SaN DieGo coUNty 5th edition Pinus torreyana subsp. torreyana Downingia concolor var. brevior Thermopsis californica var. semota Pogogyne abramsii Hulsea californica Cylindropuntia fosbergii Dudleya brevifolia Chorizanthe orcuttiana Astragalus deanei by Jon P. Rebman and Michael G. Simpson San Diego Natural History Museum and San Diego State University examples of checklist taxa: SPecieS SPecieS iNfRaSPecieS iNfRaSPecieS NaMe aUtHoR RaNk & NaMe aUtHoR Eriodictyon trichocalyx A. Heller var. lanatum (Brand) Jepson {SD 135251} [E. t. subsp. l. (Brand) Munz] Hairy yerba Santa SyNoNyM SyMBol foR NoN-NATIVE, NATURaliZeD PlaNt *Erodium cicutarium (L.) Aiton {SD 122398} red-Stem Filaree/StorkSbill HeRBaRiUM SPeciMeN coMMoN DocUMeNTATION NaMe SyMBol foR PlaNt Not liSteD iN THE JEPSON MANUAL †Rhus aromatica Aiton var. simplicifolia (Greene) Conquist {SD 118139} Single-leaF SkunkbruSH SyMBol foR StRict eNDeMic TO SaN DieGo coUNty §§Dudleya brevifolia (Moran) Moran {SD 130030} SHort-leaF dudleya [D. blochmaniae (Eastw.) Moran subsp. brevifolia Moran] 1B.1 S1.1 G2t1 ce SyMBol foR NeaR eNDeMic TO SaN DieGo coUNty §Nolina interrata Gentry {SD 79876} deHeSa nolina 1B.1 S2 G2 ce eNviRoNMeNTAL liStiNG SyMBol foR MiSiDeNtifieD PlaNt, Not occURRiNG iN coUNty (Note: this symbol used in appendix 1 only.) ?Cirsium brevistylum Cronq. indian tHiStle i checklist of the vascular plants of san Diego county 5th edition by Jon p. rebman and Michael g. simpson san Diego natural history Museum and san Diego state university publication of: san Diego natural history Museum san Diego, california ii Copyright © 2014 by Jon P. Rebman and Michael G. Simpson Fifth edition 2014. isBn 0-918969-08-5 Copyright © 2006 by Jon P. -

A Greenhouse Survey of Resistance to Frenching in Nicotiana Species
A Greenhouse Survey of Resistance to Frenching in Nicotiana Species Robert A. Steinberg Crops Research Division, Agricultural Research Service, USDA Beksville, Maryland, U.S.A. Tobacco Science, 1960, 4-47, p. 219-222, ISSN.0082-4523.pdf The data dealt with herein are on frenching b.efore removal of the in- L,evels of leaf abnormalities were re- frenchinp, a physiological disorder florescence would be satisfactory for corded as none, transient or perma- charact.eristic of, but not confined to, commercial tobacco production, since nent reticular chlorosis, and strapped Nicoticrnn (Karraker and Bortner, all new growth, i.e. all branches, are or small. Strapping had reference to 1934; Wolf, 1935). Th.e relation, if discarded. non-expansion of chlorotic leaf any, of frenching susceptibility to lamina without cessation of midrib taxonomy of Nicotiftna species is un- Experimental Methods growth. Overall leaf proportions known. The disorder appears to be The techniques employed in this were almost normal in the “small” caused by Lalloiso-leucine, a toxin study of frenching responses hav,e lype of leaf response though size was liberated by Bctcillm cerelts Frank- been previously reported (Steinberg, greatly reduced. Abnormalities in land & Frankland, a soil saphrophyte 1956). Frenchlng capacity was pro- “small” type of response included (Steinberg 1951, 19523. Soil amend- duced in the soil by liming to neu- dorsal and ventral cupping and other ments, according to Clark and Smith trality and adding a large excess of departures in leaf shape exclusive of (1949)) caused wide variations in dicalcium ph0sphat.e after steam length x width relationship. Re- counts of B. -

Flora of Australia, Volume 29, Solanaceae
FLORA OF AUSTRALIA Volume 29 Solanaceae This volume was published before the Commonwealth Government moved to Creative Commons Licensing. © Commonwealth of Australia 1982. This work is copyright. You may download, display, print and reproduce this material in unaltered form only (retaining this notice) for your personal, non-commercial use or use within your organisation. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced or distributed by any process or stored in any retrieval system or data base without prior written permission from the copyright holder. Requests and inquiries concerning reproduction and rights should be addressed to: [email protected] FLORA OF AUSTRALIA In this volume all 206 species of the family Solanaceae known to be indigenous or naturalised in Australia are described. The family includes important toxic plants, weeds and drug plants. The family Solanaceae in Australia contains 140 indigenous species such as boxthorn, wild tobacco, wild tomato, Pituri and tailflower. The 66 naturalised members include nightshade, tomato, thornapple, petunia, henbane, capsicum and Cape Gooseberry. There are keys for the identification of all genera and species. References are given for accepted names and synonyms. Maps are provided showing the distribution of nearly all species. Many are illustrated by line drawings or colour plates. Notes on habitat, variation and relationships are included. The volume is based on the most recent taxonomic research on the Solanaceae in Australia. Cover: Solanum semiarmatum F . Muell. Painting by Margaret Stones. Reproduced by courtesy of David Symon. Contents of volumes in the Flora of Australia, the faiimilies arranged according to the system of A.J.