The First Report of Root-Knot Nematode on Cestrum Nocturnum in Ninawa, Iraq
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix Color Plates of Solanales Species
Appendix Color Plates of Solanales Species The first half of the color plates (Plates 1–8) shows a selection of phytochemically prominent solanaceous species, the second half (Plates 9–16) a selection of convol- vulaceous counterparts. The scientific name of the species in bold (for authorities see text and tables) may be followed (in brackets) by a frequently used though invalid synonym and/or a common name if existent. The next information refers to the habitus, origin/natural distribution, and – if applicable – cultivation. If more than one photograph is shown for a certain species there will be explanations for each of them. Finally, section numbers of the phytochemical Chapters 3–8 are given, where the respective species are discussed. The individually combined occurrence of sec- ondary metabolites from different structural classes characterizes every species. However, it has to be remembered that a small number of citations does not neces- sarily indicate a poorer secondary metabolism in a respective species compared with others; this may just be due to less studies being carried out. Solanaceae Plate 1a Anthocercis littorea (yellow tailflower): erect or rarely sprawling shrub (to 3 m); W- and SW-Australia; Sects. 3.1 / 3.4 Plate 1b, c Atropa belladonna (deadly nightshade): erect herbaceous perennial plant (to 1.5 m); Europe to central Asia (naturalized: N-USA; cultivated as a medicinal plant); b fruiting twig; c flowers, unripe (green) and ripe (black) berries; Sects. 3.1 / 3.3.2 / 3.4 / 3.5 / 6.5.2 / 7.5.1 / 7.7.2 / 7.7.4.3 Plate 1d Brugmansia versicolor (angel’s trumpet): shrub or small tree (to 5 m); tropical parts of Ecuador west of the Andes (cultivated as an ornamental in tropical and subtropical regions); Sect. -

Green Cestrum Cestrum Parqui
Invasive plant Green cestrum Cestrum parqui Green cestrum is an escaped garden plant, which has The plant grows vigorously if neglected. On alluvial flats become a weed of roadsides, creeks and neglected sites it has been known to outcompete most other vegetation. in central and South East Queensland. The roots, seeds, stems and leaves are toxic to many domestic animals. Green cestrum needs careful control because its extensive, shallow rooty system can produce many new Generally dispersed by birds, seeds are also spread plants from suckers, particularly after root disturbance by water movement. Plants can also regrow from cut or injury. root pieces. Seedlings will not readily establish under conditions of vigorous competition with other plants. Green cestrum is poisonous to animals including cattle, Life cycle sheep, horses, pigs and poultry. Its effect on native fauna is unknown. Two alkaloids, parquine and solasonine, have Seeds germinate in autumn. Plants flower after two years been isolated from green cestrum and it is thought that and produce flow¬ers for several months through summer these substances could be responsible for its toxic effects. and autumn. Green cestrum is long-lived, producing new growth in spring. Seeds remain dormant in the soil for Symptoms in cattle include fever, loss of appetite, many years. increased thirst and eventually, general paralysis. Poultry develop acute kidney and liver damage. Post-mortem examination of poisoned animals usually reveals extensive Prevention internal haemorrhaging. Newly established plants should be destroyed before they flower and produce berries. Birds eat the berries, dispersing Time of death varies from mere hours to three days after the seed to new areas. -

Green Cestrum
OCTOBER 2008 PRIMEFACT 718 REPLACES AGFACT P7.6.44 Green cestrum Neil Griffiths Impact District Agronomist, NSW DPI, Tocal, Paterson Green cestrum is a vigorous plant that can out- compete other vegetation. Green cestrum is toxic to Dr Chris Bourke animals including cattle, sheep, horse, pigs, poultry Principal Research Scientist (Poisonous Plants), and humans. NSW DPI, Orange Agricultural Institute Habitat Green cestrum is normally found along watercourses and in non-crop areas where it usually grows in small to medium-sized thickets. Introduction Distribution Green cestrum (Cestrum parqui) is a large poisonous shrub belonging to the Solanaceae family. In NSW, green cestrum is found in the Hunter Valley, The plant is also known as green poison berry or the outer metropolitan areas of Sydney, the North Coast and the north-west, central west and south- Chilean cestrum. west of the State. Green cestrum was originally introduced into Australia from South America as an ornamental shrub for Description gardens. Since that time, it has become naturalised in Green cestrum is a medium-sized perennial areas of south-eastern Queensland, eastern New South shrub growing 2–3 m (Figure 2). It usually has many Wales (NSW) and parts of Victoria and South Australia. light-green, brittle stems. Figure 1. Green cestrum is a Class 3 noxious weed in NSW. Photo: G. Wisemantel. Leaves Fruit The shiny-green leaves are 20–30 mm wide and Clusters of shiny, black, egg-shaped berries 80–100 mm long. They have smooth edges, are 7–10 mm long are produced during summer and pointed at each end and are arranged alternately autumn (Figure 4). -

Antimicrobial Activity of the Essential Oil of Cestrum Diurnum (L.) (Solanales: Solanaceae)
African Journal of Biotechnology Vol. 4 (4), pp. 371-374, April 2005 Available online at http://www.academicjournals.org/AJB ISSN 1684–5315 © 2005 Academic Journals Short Communication Antimicrobial activity of the essential oil of Cestrum diurnum (L.) (Solanales: Solanaceae) Bhattacharjee I., Ghosh A. and Chandra G. Mosquito Research Unit, Department of Zoology, The Burdwan University, India – 713104. Accepted 22 February, 2005 Cestrum diurnum (Solanaceae: Solanales) is a single or multistemmed shrub that is also known as Day Jasmine. The essential oil of the mature leaves of C. diurnum was analyzed by GLC and GLC-MS and altogether 14 components were detected. The main constituents were palmitic acid (27.62%), stearic acid (4.62%) and oleic acid (3.06%). The essential oil of mature leaves of C. diurnum were evaluated for antimicrobial activity against pathogenic strains of Gram positive (Staphylococcus aureus, Bacillus subtilis) and Gram negative (Escherichia Coli, Pseudomonas aeruginosa) bacteria. The oil showed strong in vitro activity against P. aeruginosa and S. aureus. Key words: Cestrum diurnum, antimicrobial activity, essential oil, Gram positive bacteria, Gram negative bacteria. INTRODUCTION The development of bacterial resistance to presently shrub that is also known as Day Jasmine. There are available antibiotics has necessitated the search for new several application of the plant that have been well antibacterial agents. The gram positive bacterium such documented in several literatures and the toxicity of the as Staphylococcus aureus is mainly responsible for post species to humans and livestock has been frequently operative wound infections, toxic shock syndrome, reported (Stone, 1970; Little et al., 1974). The leaves endocarditis, osteomyelitis and food poisoning (Mylotte, contain a calcinogenic glycoside called 1,25- 1987). -

Review on Production Techniques of GI Crop, Udupi Mallige
Journal of Pharmacognosy and Phytochemistry 2018; SP3: 50-52 E-ISSN: 2278-4136 P-ISSN: 2349-8234 National conference on “Conservation, Cultivation and JPP 2018; SP3: 50-52 Utilization of medicinal and Aromatic plants" HS Chaitanya (College of Horticulture, Mudigere Karnataka, 2018) Scientist (Horticulture), Krishi Vigyan Kendra, Brahmavar, Udupi District. Karnataka, India Review on production techniques of GI Crop, Udupi Nataraja S Mallige (Jasminum sambac (L.) Aiton) Associate Professor, Dept. of Botany, Sayadhri Science College, Shivamogga District, Karnataka, India HS Chaitanya, Nataraja S, Vikram HC and Jayalakshmi Narayan Hegde Vikram HC Abstract Assistant Professor (Contract), Jasmine, Jasminum sambac (L.) Aiton cv. Udupi Mallige belonging to family Oleaceae, is a fragrant ZAHRS, Brahmavara, Udupi commercial flower crop of coastal Karnataka. Udupi Mallige is being cultivated in homestead gardens District, Karnataka, India and is concentrated in the surrounding villages of Shanakarpura, in Udupi district. The crop has been tagged under Geographical Indication (GI) due to its unique fragrance and quality flowers from Udupi Jayalakshmi Narayan Hegde region. Udupi Mallige is extensively used in religious functions and perfumery industry as it is having Associate Professor, College of Agriculture, University of mild fragrance, which gives a feeling of optimism, euphoria and confidence. Its fragrance is also known Agricultural and Horticultural to cure depression, nervous exhaustion and stress. Udupi Mallige which has been recognised Sciences, Shivamogga, internationally for its fragrance has got potential demand for export market, especially to Gulf countries. Karnataka, India The crop flowers thought the year and the peak flowering is observed during March-April (on season). There is a demand for Udupi Mallige flowers during October to February (off season), as most of the religious functions and marriage ceremonies tend to occur during off season. -

CHILEAN INKBERRY (Also Known As Green Cestrum) (Cestrum Parqui) Is an Orna- I Mental Shrub Or Small Tree That Originates from Brazil and Chile
ARC-PPRI FACT SHEETS ON INVASIVE ALIEN PLANTS AND THEIR CONTROL IN SOUTH AFRICA www.arc.agric.za CHILEAN INKBERRY (also known as green cestrum) (Cestrum parqui) is an orna- i mental shrub or small tree that originates from Brazil and Chile. It is evergreen, and generally reaches a height of 2-3 metres. The green leaves are up to 30 mm wide and 100 mm long, occur alternately on the branches, are pointed at each end (i), and produce an unpleasant odour when crushed. Greeny-yellow (ii), and sometimes purple-brown, trumpet-shaped flowers occur in axillary and terminal clusters from October to May. Alt- hough these may emit an unpleasant odour during the day, it may be quite pleasant in the evening. Flowers are followed by green berries that turn blackish (iii) as they ripen. Chilean inkberry is a category 1b declared invader in South Africa and must be con- trolled, or eradicated where possible. ii THE PROBLEM Although Chilean inkberry was originally brought into South Africa for use as a garden ornamental, it has escaped cultivation and spread through a few provinces where it is capable of invading almost any habitat. The entire plant is poisonous and has been known to kill livestock, and even humans who ate parts of it. However, the ripe berries are readily consumed by birds that aid in seed dispersal. This fact is evident even in suburban gardens, especially in Gauteng, where Chilean inkberry plants seem to appear from nowhere and are difficult to eradicate because they merely sucker from the roots when cut down. -

Delicate, Fragrant, Lady of the Night- a Medicinal Received: 04-09-2016 Accepted: 10-10-2016 Gift
Journal of Medicinal Plants Studies 2016; 4(6): 13-17 ISSN 2320-3862 JMPS 2016; 4(6): 13-17 © 2016 JMPS Delicate, fragrant, lady of the night- A medicinal Received: 04-09-2016 Accepted: 10-10-2016 gift Amin Shaista Department of Pharmaceutical Amin Shaista and Parle Amrita Chemistry, Delhi Pharmaceutical Science and Research University, Abstract New Delhi India Night blooming jasmine, botanically known as Cestrum nocturnum is an evergreen shrub that grows in Parle Amrita tropical and sub-tropical regions throughout the world. Cestrum nocturnum is a popular ornamental plant Department of Pharmaceutical due to its showy and fragrant white flowers. It is also used as a hedge plant and cultivated as a medicinal Chemistry, Delhi Pharmaceutical plant. The medicinal properties of night blooming jasmine include antioxidant, anti-hyperlipidemic, Science and Research University, hepatoprotective, analgesic, antibacterial, antifungal, anti-convulsant, anti-HIV and larvicidal activities. New Delhi India The present paper reviews the geographical distribution, history, cultivation, uses, side effects, synonyms, botanical description, taxonomical classification, phytochemical constituents and pharmacological activities. Keywords: Cestrum nocturnum, antibacterial, antioxidant, anti-inflammatory, larvicidal Introduction Cestrum nocturnum is a garden shrub from the family Solanaceae, commonly known as "lady of the night" which is used as a remedy for different health disorders. This sprawling shrub has glossy simple leaves, vine like stems, greenish-creamy white tubular flowers and fleshy berries. The berries are marfil white or aubergine in colour. The species name ‘nocturnum’ refers to the species’ habit of opening its small, heavily-scented flowers at night. The flowers release powerful sweet perfume at night. It is made into a rare attar (raat ki rani) which is used in Indian and Middle East perfumery. -
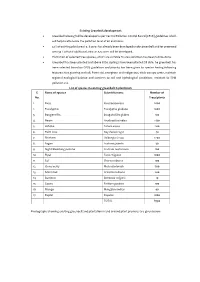
CPCB) Guidelines Which Will Help to Attenuate the Pollution Level of Air and Noise
Existing Greenbelt development Greenbelt is being/will be developed as per Central Pollution Control Board (CPCB) guidelines which will help to attenuate the pollution level of air and noise. 33 % of existing plant area i.e. 8 acres has already been developed under greenbelt and for proposed area 33 % of total additional area i.e. 5.5 acres will be developed. Plantation of selected tree species, which are suitable to area condition has been/will be done. Greenbelt has been planted and above 8750 saplings have been planted till date. he greenbelt has been selected based on CPCB guidelines and priority has been given to species having following features; fast growing and tall, Perennial, evergreen and indigenous, thick canopy cover, maintain regional ecological balance and conform to soil and hydrological conditions, resistant to SPM pollution etc. List of species in existing greenbelt & plantation S. Name of species Scientific name Number of No. Trees/plants 1. Ficus Ficus benjamina 2000 2. Eucalyptus Eucalyptus globulus 1000 3. Bougenvillia Bougainvillea glabra 100 4. Neem Azadirachta indica 1130 5. Ashoka Saraca asoca 200 6. Palm Tree Roystonea regia 40 7. Shisham Dalbergia sissoo 1200 8. Sagon Tectona grandis 30 9. Night blooming jasmine Cestrum nocturnum 100 10. Pipal Ficus religiosa 1000 11. Sal Shorea robusta 100 12. China berry Melia azedarach 500 13. Silver Oak Grevillea robusta 200 14. Bamboo Bambusa vulgaris 10 15. Guava Psidium guajava 100 16. Mango Mangifera indica 40 17. Poplar Populus 1000 TOTAL 8750 Photographs showing existing greenbelt and plantation in and around plant premises are given below: Action plan for greenbelt development for proposed area The rate of pollutant removal is found to increase linearly as the concentration of the pollutant increases over the range of concentration that are encountered in ambient air and which are low enough not to cause stomatal closure. -

Cestrum Nocturnum Global Invasive
FULL ACCOUNT FOR: Cestrum nocturnum Cestrum nocturnum System: Terrestrial Kingdom Phylum Class Order Family Plantae Magnoliophyta Magnoliopsida Solanales Solanaceae Common name onaona Iapana (Hawaiian), ike he po (Niuean), thauthau (Fijian), ali'I o le po (Samoan), teine 'o le po (Samoan), galan de noche (Spanish), ye xiang shu (Mandarin), laukau po'uli (Tongan), fafine o te po (Tuvaluan), ala aumoe (Hawaiian), kupaoa (Hawaiian), thauthau ni mbongi (Fijian), tiare ariki va'ine (Cook Islands), jonoul ruo awa (Marshallese), ai pua e pogi (Rotuman), ariki-va'ine (Cook Islands), night jessamine (English), kara (Fijian), dama de noche (Chamorro), night queen (English), lady of the night (English), night cestrum (English), night-blooming jasmine (English), night-flowering cestrum (English), dama di noche (Chamorro), night-flowering jasmine (English), queen of the night (English) Synonym Cestrum parqui Similar species Cestrum parqui Summary Cestrum nocturnum commonly known as queen of the night, is a popular ornamental species widely distributed for its strongly fragrant flowers that bloom at night. Having bird-dispersed seeds and the ability to reproduce vegetatively has resulted in escapes from cultivation, where in some areas it aggressively colonises disturbed sites such as road edges and forest gaps forming dense impenetrable thickets and resulting in competition with and displacement of native plant species. C. nocturnum is also known to be poisonous if ingested, forming a risk to grazing livestock and has been known to produce hay-fever like symptoms in some people. view this species on IUCN Red List Global Invasive Species Database (GISD) 2021. Species profile Cestrum nocturnum. Pag. 1 Available from: http://www.iucngisd.org/gisd/species.php?sc=851 [Accessed 07 October 2021] FULL ACCOUNT FOR: Cestrum nocturnum Species Description Cestrum nocturnum is a glabrous shrub that grows from 1 to 5 m tall (depending on location) with ovate-oblong, petiolate, and obtuse leaves mostly 7 - 20 cm long (Webb et al., 1988; Tharman et al., 1994; Zhang et al., 1994). -

Antimicrobial Activity of Cestrum Aurantiacum L
Int.J.Curr.Microbiol.App.Sci (2015) 4(3): 830-834 ISSN: 2319-7706 Volume 4 Number 3 (2015) pp. 830-834 http://www.ijcmas.com Original Research Article Antimicrobial Activity of Cestrum aurantiacum L. B.Sivaraj, C.Vidya, S.Nandini and R.Sanil* Department of Zoology, Government Arts College, Udhagamandalam 643002, The Nilgiris, India *Corresponding author A B S T R A C T Antimicrobial studies were conducted in the Cestrum aurentiacum which is K e y w o r d s commonly called orange Cestrum, an exotic plant common in the Nilgiris. The plant material was collected fresh from the Nilgiris and the root, the leaf and the Cestrum, flower were separated. All these plant materials were dried at 37oC and extracted in Antimicrobial ethyl alcohol. Antimicrobial activity of the extract was carried out against activity, Kliebsella, Proteus, Staphylococcus, E. coli, & Pseudomonas. Among the extract Proteus, of various plant parts, the flower extract shows maximum antimicrobial activity. Pseudomonas The report that the Cestrum aurentiacum can be used as an antimicrobial agent is a new one. The active principle behind this may be alkaloid or saponin, but to prove this, more study has to be conducted. Introduction Cestrum aurantiacum L. (Capraria large acutish lober strongly reflexed. It is lanceolata), also called orange Cestrum, claimed to be a poisonous plant and all the orange Jessamine, orange flowering plant part if ingested is considered to be Jessamine and yellow Cestrum) is an poisonous. However, it attracts a lot of bees, invasive species native to North and South birds etc. The studies in the related species America belongs to Solanacea family. -

The Way of Tea
the way of tea | VOLUME I the way of tea 2013 © CHADO chadotea.com 79 North Raymond Pasadena, CA 91103 626.431.2832 DESIGN BY Brand Workshop California State University Long Beach art.csulb.edu/workshop/ DESIGNERS Dante Cho Vipul Chopra Eunice Kim Letizia Margo Irene Shin CREATIVE DIRECTOR Sunook Park COPYWRITING Tek Mehreteab EDITOR Noah Resto PHOTOGRAPHY Aaron Finkle ILLUSTRATION Erik Dowling the way of tea honored guests Please allow us to make you comfortable and serve a pot of tea perfectly prepared for you. We also offer delicious sweets and savories and invite you to take a moment to relax: This is Chado. Chado is pronounced “sado” in Japanese. It comes from the Chinese words CHA (“tea”) and TAO (“way”) and translates “way of tea.” It refers not just to the Japanese tea ceremony, but also to an ancient traditional practice that has been evolving for 5,000 years or more. Tea is quiet and calms us as we enjoy it. No matter who you are or where you live, tea is sure to make you feel better and more civilized. No pleasure is simpler, no luxury less expensive, no consciousness-altering agent more benign. Chado is a way to health and happiness that people have loved for thousands of years. Thank you for joining us. Your hosts, Reena, Devan & Tek A BRIEF HISTORY OF CHADO Chado opened on West 3rd Street in 1990 as a small, almost quaint tearoom with few tables, but with 300 canisters of teas from all over the globe lining the walls. In 1993, Reena Shah and her husband, Devan, acquired Chado and began quietly revolutionizing how people in greater Los Angeles think of tea. -

See Below for Price List
See below for price list. PROD ID Description Common Name Size Price 26900 Agapanthus 'Blue Yonder' African Lily P10 $ 29.99 13941 Agave Century Plant P4.5 $ 14.99 14526 Allamanda Bush Allamanda Bush P10 $ 25.00 20379 Alocasia 'Borneo Giant' Elephant's Ear P10 $ 29.99 27645 Aloe polyphylla Spiral Aloe C2 $ 39.99 13340 Ananas comosus Pineapple P6 $ 29.99 24715 Bougainvillea (Bush Form) Bougainvillea Bush P10 $ 25.00 28434 Bougainvillea (Hanging Basket) Bouganvillea HB12 $ 29.99 26290 Bouganvilla on Trellis Bougainvillea P10 trellis $ 29.99 17303 Canna indica Canna Lily C1 $ 14.99 25705 Canna indica Canna Lily C2 $ 19.99 19403 Cordyline australis 'Red Sensation' Red Sensation Cordyline P10 $ 29.99 20340 Cordyline australis 'Red Sensation' Red Sensation Cordyline P6 $ 16.99 20641 Cordyline australis 'Red Star' Red Star Cordyline P10 $ 29.99 20341 Cordyline australis 'Red Star' Red Star Cordyline P6 $ 16.99 26911 Crossandra infundibuliformis Crossandra P10 $ 25.00 13831 Cuphea hyssopifolia Mexican Heather P6.5 $ 6.99 28987 Cycas revoluta Sago Palm C1 $ 29.99 8261 Cycas revoluta Sago Palm P10 $ 39.99 13658 Cyclamen persicum Cyclamen P6.5 $ 10.99 23465 Cymbopogon citratus Lemon Grass P6 $ 10.99 20642 Cyperus 'Little Tut' Dwarf Egyption Papyrus P10 $ 25.00 20339 Dipladenia boliviensis Dipladenia HB12 $ 29.99 25678 Dipladenia boliviensis (Bush Form) Bush Dipladenia P10 $ 29.99 25685 Dipladenia boliviensis 'Coral' (Bush Form) Coral Dipladenia P10 $ 29.99 26208 Dipladenia boliviensis 'Coral' (Bush Form) Coral Dipladenia P6 $ 16.99 28967 Dipladenia