Blends of Polycarbonate Containing Fluorinated-Bisphenol-A and Polyvinyl Chloride
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polycarbonate Lenses
Polycarbonate Lenses The most impact resistant of all lens materials is polycarbonate. Children, athletes, anyone working at a job or hobby where they might get hit in the face and need safety glasses, people with only one eye, those who fall a lot, are natural candidates for polycarbonate lenses. If safety is a prime concern, choose polycarbonate lenses. Advantages of polycarbonate lenses : 1. Polycarbonate has four to five times the impact resistance of glass or plastic. When glass or plastic lenses break, they do not break into harmless granules, but can break into sharp shards that can enter your eye and destroy your vision. Poly- carbonate is far and away the safest of all the lenses made. 2. Polycarbonate is the lightest lens material made. 3. Polycarbonate lenses naturally provide protection against ultra-violet light, at no additional charge. 4. Polycarbonate lenses come with a scratch resistant coating (not scratch proof) at no additional charge. 5. Polycarbonate is a high index material, so the lenses will be thinner than if made with glass or plastic. Disadvantages of polycarbonate lenses : 1. People in prescriptions with higher powers sometimes have trouble seeing out the edges of the lenses--your clear field of vision is not as wide as with glass or plas- tic lenses. The lenses are made with different curves than are used to make the same pre- scription power in glass or plastic, so you will see out of these lenses a little dif- ferently. People with prescriptions up to plus or minus three diopters (most people) usually have no problem adjusting to polycarbonate lenses. -

Migration of Bisphenol a from Polycarbonate Plastic of Different Qualities
Migration of bisphenol A from polycarbonate plastic of different qualities Environmental project No. 1710, 2015 [Series Title and year] Title: Editing: Migration of Bisphenol A from polycarbonate Gitte Alsing Pedersen, DTU National Food Institute, plastic of different qualities Søren Hvilsted, DTU Danish Polymer Centre, Department of Chemical and Biochemical Engineering and Jens Højslev Petersen, DTU National Food Institute Technical University of Denmark Published by: The Danish Environmental Protection Agency Strandgade 29 1401 Copenhagen K Denmark www.mst.dk/english Year: ISBN no. 2015 978-87-93352-24-7 Disclaimer: When the occasion arises, the Danish Environmental Protection Agency will publish reports and papers concerning research and development projects within the environmental sector, financed by study grants provided by the Danish Environmental Protection Agency. It should be noted that such publications do not necessarily reflect the position or opinion of the Danish Environmental Protection Agency. However, publication does indicate that, in the opinion of the Danish Environmental Protection Agency, the content represents an important contribution to the debate surrounding Danish environmental policy. Sources must be acknowledged. 2 Migration of Bisphenol A from polycarbonate plastic of different qualities Contents Foreword .................................................................................................................. 5 Conclusion and Summary ......................................................................................... -

United States Patent (19) 11 Patent Number: 4,481,333 Fleischer Et Al
United States Patent (19) 11 Patent Number: 4,481,333 Fleischer et al. 45 Date of Patent: Nov. 6, 1984 54 THERMOPLASTIC COMPOSITIONS 58 Field of Search ................................ 525/192, 199 COMPRISING WINYL CHLORIDE POLYMER, CLPE AND FLUOROPOLYMER 56) References Cited U.S. PATENT DOCUMENTS 75) Inventors: Dietrich Fleischer, Darmstadt; Eckhard Weber, Liederbach; 3,005,795 10/1961 Busse et al. ......................... 525/199 3,294,871 2/1966 Schmitt et al. ...... 52.5/154 X Johannes Brandrup, Wiesbaden, all 3,299,182 1/1967 Jennings et al. ... ... 525/192 of Fed. Rep. of Germany 3,334,157 8/1967 Larsen ..................... ... 525/99 73 Assignee: Hoechst Aktiengesellschaft, Fed. 3,940,456 2/1976 Fey et al. ............................ 525/192 Rep. of Germany Primary Examiner-Carman J. Seccuro (21) Appl. No.: 566,207 Attorney, Agent, or Firm-Connolly & Hutz 22 Filed: Dec. 28, 1983 57 ABSTRACT 30 Foreign Application Priority Data The invention relates to a thermoplastic composition which comprises vinyl chloride polymers and chlori Dec. 31, 1982 (DE Fed. Rep. of Germany ....... 3248.731 nated polyethylene and which contains finely divided 51) Int. Cl. ...................... C08L 23/28; C08L 27/06; fluoropolymers and has a markedly improved process C08L 27/18 ability, particularly when shaped by extrusion. 52 U.S. C. .................................... 525/192; 525/199; 525/239 7 Claims, No Drawings 4,481,333 2 iaries and do not provide a solution to the present prob THERMOPLASTC COMPOSITIONS lem. COMPRISINGVINYL CHLORIDE POLYMER, -

Toxicity of the Pyrolysis and Combustion Products of Poly (Vinyl Chlorides): a Literature Assessment
FIRE AND MATERIALS VOL. II, 131-142 (1987) Toxicity of the Pyrolysis and Combustion Products of Poly (Vinyl Chlorides): A Literature Assessment Clayton Huggett and Barbara C. Levin* us Department of Commerce, National Bureau of Standards, National Engineering Laboratory, Center for Fire Research, Gaithersburg, MD 20899, USA Poly(vinyl chlorides) (PVC) constitute a major class of synthetic plastics. Many surveys of the voluminous literature have been performed. This report reviews the literature published in English from 1969 through 1984 and endeavors to be more interpretive than comprehensive. pve compounds, in general, are among the more fire resistant common organic polymers, natural or synthetic. The major products of thermal decomposition include hydrogen chloride, benzene and unsaturated hydrocarbons. In the presence of oxygen, carbon monoxide, carbon dioxide and water are included among the common combustion products. The main toxic products from PVC fires are hydrogen chloride (a sensory and pulmonary irritant) and carbon monoxide (an asphyxiant). The LCso values calculated for a series of natural and synthetic materials thermally decomposed according to the NBS toxicity test method ranged from 0.045 to 57 mgl-l in the flaming mode and from 0.045 to > 40mgl-l in the non-flaming mode. The LCso results for a PVC resin decomposed under the same conditions were 17 mg 1- 1 in the flaming mode and 20 mg 1- 1 in the non-flaming mode. These results indicate that PVC decomposition products are not extremely toxic when compared with those from other common building materials. When the combustion toxicity (based on their HCI content) of PVC materials is compared with pure HCI experiments, it appears that much of the post-exposure toxicity can be explained by the HCI tha t is genera ted. -

Study of Surface Mechanical Characteristics of ABS/PC Blends Using Nanoindentation
processes Article Study of Surface Mechanical Characteristics of ABS/PC Blends Using Nanoindentation Saira Bano 1, Tanveer Iqbal 2, Naveed Ramzan 3 and Ujala Farooq 4,* 1 Department of Chemical & Polymer Engineering, University of Engineering & Technology, FSD Campus, Lahore 38000, Pakistan; [email protected] 2 Department of Chemical, Polymer & Composite Materials Engineering, University of Engineering & Technology, KSK Campus, Lahore 54890, Pakistan; [email protected] 3 Department of Chemical Engineering, University of Engineering & Technology, KSK Campus, Lahore 54890, Pakistan; [email protected] 4 Faculty of Aerospace Engineering, Aerospace Manufacturing Technologies, Delft University of Technology, Kluyverweg 1, 2629 HS Delft, The Netherlands * Correspondence: [email protected] Abstract: Acrylonitrile butadiene styrene (ABS) and polycarbonate (PC) are considered a well-known class of engineering thermoplastics due to their efficient use in automotive, 3D printing, and elec- tronics. However, improvement in toughness, processability, and thermal stability is achieved by mixing together ABS and PC. The present study focuses on the understanding of surface mechani- cal characterization of acrylonitrile butadiene styrene (ABS) and polycarbonate (PC) blends using nano-indentation. Polymer blends sheets with three different proportions of ABS/PC (75:25, 50:50, and 25:75) were fabricated via melt-processing and thermal press. Fourier transform infrared (FTIR) spectroscopy was performed to analyze the intermolecular interactions between the blends’ compo- nents. To understand the surface mechanical properties of ABS and PC blends, a sufficient number Citation: Bano, S.; Iqbal, T.; Ramzan, of nano-indentation tests were performed at a constant loading rate to a maximum load of 100 mN. N.; Farooq, U. -

Effects of Polypropylene, Polyvinyl Chloride, Polyethylene Terephthalate, Polyurethane, High
Effects of polypropylene, polyvinyl chloride, polyethylene terephthalate, polyurethane, high- density polyethylene, and polystyrene microplastic on Nelumbo nucifera (Lotus) in water and sediment Maranda Esterhuizen ( maranda.esterhuizen@helsinki. ) University of Helsinki: Helsingin Yliopisto https://orcid.org/0000-0002-2342-3941 Youngjun Kim Korea Institute of Science and Technology Europe Forschungsgesellschaft mbH Research Article Keywords: Microplastics, oxidative stress, sediment, macrophyte, exposure, germination, seedling growth Posted Date: May 11th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-458889/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Loading [MathJax]/jax/output/CommonHTML/jax.js Page 1/20 Abstract Plastic waste is recognised as hazardous, with the risk increasing as the polymers break down in nature to secondary microplastics or even nanoplastics. The number of studies reporting on the prevalence of microplastic in every perceivable niche and bioavailable to biota is dramatically increasing. Knowledge of the ecotoxicology of microplastic is advancing as well; however, information regarding plants, specically aquatic macrophytes, is still lacking. The present study aimed to gain more information on the ecotoxicological effects of six different polymer types as 4 mm microplastic on the morphology (germination and growth) and the physiology (catalase and glutathione S-transferase activity) of the rooted aquatic macrophyte, Nelumbo nucifera. The role of sediment was also considered by conducting all exposure both in a sediment-containing and sediment-free exposure system. Polyvinyl chloride and polyurethane exposures caused the highest inhibition of germination and growth compared to the control. However, the presence of sediment signicantly decreased the adverse effects. -

Miscibility Studies, Characterization and Properties Evaluation of Pvc/Pmma Blends
International Journal of Scientific & Engineering Research, Volume 7, Issue 8, August-2016 1766 ISSN 2229-5518 MISCIBILITY STUDIES, CHARACTERIZATION AND PROPERTIES EVALUATION OF PVC/PMMA BLENDS. Chandramohan, B.Rajam, V.V.Basava Rao Abstract: Poly Vinyl Chloride (PVC) and Poly methyl methacrylate (PMMA) blends are prepared by injection moulding method over an entire composition range of 0/100, 25/75, 50/50, 75/25 and 100/0 by weight. The miscibility of the blends was characterized by dynamic mechanical thermal analysis. The effect of polymer-polymer interactions on the miscibility of PVC/PMMA blends were studied using the method. The effect of blending poly (methyl methacrylate) in various proportions with suitably stabilized and plasticized poly (vinyl chloride) was studied with reference to their physical, mechanical, and thermal properties. Distribution of the phases in the blends will be studied through scanning electron microscopy. These properties of PVC/PMMA blends are correlated with spectroscopic investigation. Keywords: Poly Vinyl Chloride, Poly Methyl Methacrylate, dynamic mechanical analysis, Morphology studies, Injection moulding —————————— —————————— 1.0 Introduction: Poly vinyl chloride (PVC) is a normal impact, high corrosion resistant thermoplastic. Due to the exceptional corrosion resistance of the material, it is used where maximum chemical resistance is necessary. The low cost is also one favourable factor in choosing the compound for various applications in industries and in domestic usage. Approximately 75% of the world’s PVC is produced by the suspension polymerization (S- PVC) process inIJSER which the polymerization is carried out inside vinyl chloride monomer (VCM) droplets dispersed in water. The product is in the form of porous 100-150 µm- diameter grains. -
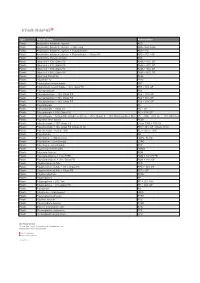
Type Material Name Abbreviation Plastic Acrylonitrile Butadiene
Type Material Name Abbreviation Plastic Acrylonitrile butadiene styrene ABS Plastic Acrylonitrile butadiene styrene - High-Temp ABS - high temp Plastic Acrylonitrile butadiene styrene + Polycarbonate ABS + PC Plastic Acrylonitrile butadiene styrene + Polycarbonate + Glass Fill ABS + PC + GF Plastic Acrylonitrile styrene acrylate ASA Plastic Nylon 6-6 + 10% Glass Fill PA66 + 10% GF Plastic Nylon 6-6 + 20% Glass Fill PA66 + 20% GF Plastic Nylon 6-6 + 30% Glass Fill PA66 + 30% GF Plastic Nylon 6-6 + 50% Glass Fill PA66 + 50% GF Plastic Nylon 6-6 Polyamide PA66 Plastic Polyamide 12 PA12 Plastic Polybutylene terephthalate PBT Plastic Polybutylene terephthalate + 30% Glass Fill PBT+ 30% GF Plastic Polycaprolactam PA6 Plastic Polycaprolactam + 20% Glass Fill PA6 + 20% GF Plastic Polycaprolactam + 30% Glass Fill PA6 + 30% GF Plastic Polycaprolactam + 50% Glass Fill PA6 + 50% GF Plastic Polycarbonate PC Plastic Polycarbonate + Glass Fill PC + GF Plastic Polycarbonate + 10% Glass Fill PC + 10% GF Plastic Polycarbonate + Acrylonitrile butadiene styrene + 20% Glass Fill + 10% Stainless Steel fiber PC + ABS + 20% GF + 10% SS Fiber Plastic Polyether ether ketone PEEK Plastic Polyetherimide + 30% Glass Fill Ultem 1000 + 30% GF Plastic Polyetherimide + 40% Glass Fill (Ultem 2410) PEI + 40% GF (Ultem 2410) Plastic Polyetherimide + Ultem 1000 PEI + Ultem 1000 Plastic Polyethylene PE Plastic Polyethylene - High-Density HDPE, PEHD Plastic Polyethylene - Low-Density LDPE Plastic Polyethylene terephthalate PET Plastic Polymethyl methacrylate PMMA Plastic Polyoxymethylene -

Environmental Impacts of Polyvinyl Chloride Building Materials
Environmental Impacts of Polyvinyl Chloride Building Materials by Joe Thornton, Ph.D. A Healthy Building Network Report Environmental Impacts of Polyvinyl Chloride Building Materials by Joe Thornton, Ph.D. A Healthy Building Network Report This report was prepared by the author and does not represent the opinions of The University of Oregon or any of its affiliates. © Healthy Buiding Network, 2002 Washington, D.C. The United States ISBN 0-9724632-0-8 Printed in the United States of America on acid-free, recycled paper, using soy-based ink. Photo Credits: p. 1 © Greenpeace p. 41 © Leuders/Greenpeace. p. 53 © Basel Action Network. PVC coated wire waste sorted and burned by villagers in Guiyu, China. Cancer causing polycyclic aro- matic hydrocarbons and dioxins will result from burning wires made from PVC and brominated flame retardants. p. 77 © Bryce lankard/Greenpeace 1996. Cows graze outside PVC manufacturing facility in Giesmar, Louisiana. Dioxin emissions to the environment move up the food chain. Fatty foods such as meat and dairy products are the primary source of dioxin exposure to most humans. p. 97 © Stone/Greenpeace 200. Lake Charles, Louisiana area children suffer health impacts due to pollution from PVC manufacturing facilities. Acknowledgements The Healthy Building acknowledges the following people and organiza- tions for their important contributions to this report. Dr. Joe Thornton’s willingness to accept this assignment on short notice and under a short deadline is testament to his ongoing commitment to the related goals -

Monomers – Styrene and Vinyl Chloride
Chemical Information Sheet Version 2.0 | March 2021 MONOMERS – STYRENE AND VINYL CHLORIDE Other Names Styrene: Ethenylbenzene, vinylbenzene, Monomers are chemical precursors that link together to phenylethene create polymer materials. Styrene and vinyl chloride are Vinyl Chloride: VCM, chloroethene monomers that may be present in low concentrations in some polymer materials. The presence of these monomers can be related to the process controls during CAS Number Substance polymer production. 100-42-5 Styrene Uses in the Supply Chain 75-01-4 Vinyl Chloride Styrene is a colorless liquid that evaporates easily which may be used to create polymers including polystyrene, ABS plastic, May Be Found In Styrene: Polystyrene, Acrylonitrile-butadiene- synthetic rubber (SBR) and other materials. Styrene can also be styrene (ABS) plastic, Styrene-butadiene rubber (SBR), styrene-divinylbenzene (S-DVB) used in plastic packaging and electrical parts. Vinyl Chloride: Polyvinyl chloride (PVC), Vinyl Chloride is used in production of polyvinyl chloride vinyl polymers, plastisol screen prints, plastic (PVC) and vinyl polymers, which can be hard or flexible parts, coatings for leather, synthetic leather and materials. PVC can be associated with plastisol screen prints, textiles plastic parts, and a variety of coatings on leather, synthetic leather, and textiles. Why Monomers Are Restricted ▪ Legislation in major markets globally restricts or regulates the presence of styrene and vinyl chloride in finished products or materials. ▪ Monomers can present a variety -

Interaction Between Plasticized Polyvinyl Chloride Waterproofing Membrane and Extruded Polystyrene Board, in the Inverted Flat Roof
MATERIALES DE CONSTRUCCIÓN Vol. 64, Issue 316, October–December 2014, e037 ISSN-L: 0465-2746 http://dx.doi.org/10.3989/mc.2014.008913 Interaction between plasticized polyvinyl chloride waterproofing membrane and extruded polystyrene board, in the inverted flat roof A. Pedrosa *, M. Del Río, C. Fonseca Universidad Politécnica de Madrid (Madrid, Spain) *[email protected] Received 22 October 2013 Accepted 6 June 2014 Available on line 2 December 2014 ABSTRACT: The inverted flat roof is a constructive system widely used in flat roof construction. In this con- structive solution, the insulation is placed over the waterproofing material as a protection. It is believed that this solution provides a longer life cycle; given the fact that it limits the thermal variation the waterproofing material bears up to the end of its life cycle. Consequently, the result will be providing a longer life to the waterproofing membrane. This constructive solution always incorporates polymers or other materials with a thermoplastic addition in their composition. Some polymers show interactions between them that can affect their integrity, and, at the same time, the bulk of the polymeric materials are incompatible. The extruded polystyrene board is always present in the inverted flat roof, and although it is an unbeatable product for this use, it presents incompatibilities and interactions with other materials, and these can affect their properties and therefore the durability of them. KEYWORDS: Inverted flat roof; Extruded polystyrene board; Waterproofing; Plasticizers migration; Single ply membrane Citation / Citar como: Pedrosa, A.; Del Río, M.; Fonseca, C. (2014) Interaction between plasticized polyvinyl chloride waterproofing membrane and extruded polystyrene board, in the inverted flat roof. -

Polycarbonate (PC)
Polycarbonate (PC) Carlos Buitrago Introduction to Polymers CE 435 OVERVIEW zIntroduction zStructure of PC zSynthesis zManufacturing zPhysical Properties of PC zApplications of PC zPC Blends zQuestions INTRODUCTION z Polycarbonate (PC), was first developed in 1953 by Bayer in Germany, and General Electric in the US independently. Its most popular trade name is LEXAN® z PC is one of the high performance heterochain polymeric materials that comprise the family of “engineering thermoplastics” z PC is a good material choice in industry not only due to its characteristics, but also because its processing is environmentally friendly, and it can be recycled STRUCTURE OF PC z A polycarbonate molecule is composed by a Bisphenol A part and a carbonate group z Bisphenol A contains two aromatic rings, which are responsible for PC’s stiff LG-DOW (n.d.). Molecular structure of polycarbonate. backbone http://www.lg-dow.com/tech/tech.htm z The Bisphenol A group also • The Characteristic high contributes to PC’s inability glass transition temperature to crystallize. This (Tg = 145ºC) of PC is caused amorphous structure gives by the minimal molecular the polymer its particular rotation about the bonds transparency SYNTHESIS PC is most often synthesized from Bisphenol A and phosgene by a step-growth polymerization in which Cl- ions are eliminated every time the monomers react. This kind of step-growth polymerization is often called a condensation process Polymerization Steps 1. The Bisphenol A groups are reacted with proton acceptors such as NaOH to obtain the polymerization functional groups Polymerization steps (Cont’d) 2. The deprotonated Bispohenol A reacts with Phosgene and a catalyst at temperatures between 25 and 35ºC.