Miscibility Studies, Characterization and Properties Evaluation of Pvc/Pmma Blends
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blends of Polycarbonate Containing Fluorinated-Bisphenol-A and Polyvinyl Chloride
Europaisches Patentamt European Patent Office © Publication number: 0 576 057 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 93201533.2 int. Ci.5; C08L 69/00, C08L 27/06, C08G 64/10, //(C08L69/00, @ Date of filing: 28.05.93 27:06),(C08L27/06,69:00) © Priority: 01.06.92 US 891032 © Applicant: ENICHEM S.p.A. Piazza della Repubblica, 16 @ Date of publication of application: 1-20124 Milano(IT) 29.12.93 Bulletin 93/52 @ Inventor: Drzewinski, Michael A. © Designated Contracting States: 371 Clarksville Road, Princeton Junction AT BE CH DE DK ES FR GB GR IE IT LI LU MC New Jersey 08850(US) NL PT SE © Representative: Roggero, Sergio et al Ing. Barzano & Zanardo Milano S.p.A. Via Borgonuovo 10 1-20121 Milano (IT) © Blends of polycarbonate containing fluorinated-bisphenol-A and polyvinyl chloride. © Bisphenol A polycarbonate containing at least 15 mole % of 2,2-bis-(4-hydroxyphenyl)hexafluoropropane (6F-Bisphenol A) can be blended with polyvinyl chloride (PVC) to form a thermodynamically miscible, transpar- ent, single phase blend at all compositions. Such blends are flame resistant as well as resistant to attack by acids, bases and many organic solvents. CO Rank Xerox (UK) Business Services (3. 10/3.6/3.3. 1) EP 0 576 057 A1 BACKGROUND OF THE INVENTION Field of the Invention: 5 This invention pertains to mixtures of polyvinyl chloride (PVC) and polycarbonates which contain at least 15 mole % of fluorinated bisphenol monomer units (F-PC) such as 2,2-bis-(4-hydroxyphenyl)- hexafluoropropane (6F-bisphenol A), herein referred to as 6F-PC. -

United States Patent (19) 11 Patent Number: 4,481,333 Fleischer Et Al
United States Patent (19) 11 Patent Number: 4,481,333 Fleischer et al. 45 Date of Patent: Nov. 6, 1984 54 THERMOPLASTIC COMPOSITIONS 58 Field of Search ................................ 525/192, 199 COMPRISING WINYL CHLORIDE POLYMER, CLPE AND FLUOROPOLYMER 56) References Cited U.S. PATENT DOCUMENTS 75) Inventors: Dietrich Fleischer, Darmstadt; Eckhard Weber, Liederbach; 3,005,795 10/1961 Busse et al. ......................... 525/199 3,294,871 2/1966 Schmitt et al. ...... 52.5/154 X Johannes Brandrup, Wiesbaden, all 3,299,182 1/1967 Jennings et al. ... ... 525/192 of Fed. Rep. of Germany 3,334,157 8/1967 Larsen ..................... ... 525/99 73 Assignee: Hoechst Aktiengesellschaft, Fed. 3,940,456 2/1976 Fey et al. ............................ 525/192 Rep. of Germany Primary Examiner-Carman J. Seccuro (21) Appl. No.: 566,207 Attorney, Agent, or Firm-Connolly & Hutz 22 Filed: Dec. 28, 1983 57 ABSTRACT 30 Foreign Application Priority Data The invention relates to a thermoplastic composition which comprises vinyl chloride polymers and chlori Dec. 31, 1982 (DE Fed. Rep. of Germany ....... 3248.731 nated polyethylene and which contains finely divided 51) Int. Cl. ...................... C08L 23/28; C08L 27/06; fluoropolymers and has a markedly improved process C08L 27/18 ability, particularly when shaped by extrusion. 52 U.S. C. .................................... 525/192; 525/199; 525/239 7 Claims, No Drawings 4,481,333 2 iaries and do not provide a solution to the present prob THERMOPLASTC COMPOSITIONS lem. COMPRISINGVINYL CHLORIDE POLYMER, -

Toxicity of the Pyrolysis and Combustion Products of Poly (Vinyl Chlorides): a Literature Assessment
FIRE AND MATERIALS VOL. II, 131-142 (1987) Toxicity of the Pyrolysis and Combustion Products of Poly (Vinyl Chlorides): A Literature Assessment Clayton Huggett and Barbara C. Levin* us Department of Commerce, National Bureau of Standards, National Engineering Laboratory, Center for Fire Research, Gaithersburg, MD 20899, USA Poly(vinyl chlorides) (PVC) constitute a major class of synthetic plastics. Many surveys of the voluminous literature have been performed. This report reviews the literature published in English from 1969 through 1984 and endeavors to be more interpretive than comprehensive. pve compounds, in general, are among the more fire resistant common organic polymers, natural or synthetic. The major products of thermal decomposition include hydrogen chloride, benzene and unsaturated hydrocarbons. In the presence of oxygen, carbon monoxide, carbon dioxide and water are included among the common combustion products. The main toxic products from PVC fires are hydrogen chloride (a sensory and pulmonary irritant) and carbon monoxide (an asphyxiant). The LCso values calculated for a series of natural and synthetic materials thermally decomposed according to the NBS toxicity test method ranged from 0.045 to 57 mgl-l in the flaming mode and from 0.045 to > 40mgl-l in the non-flaming mode. The LCso results for a PVC resin decomposed under the same conditions were 17 mg 1- 1 in the flaming mode and 20 mg 1- 1 in the non-flaming mode. These results indicate that PVC decomposition products are not extremely toxic when compared with those from other common building materials. When the combustion toxicity (based on their HCI content) of PVC materials is compared with pure HCI experiments, it appears that much of the post-exposure toxicity can be explained by the HCI tha t is genera ted. -

Effects of Polypropylene, Polyvinyl Chloride, Polyethylene Terephthalate, Polyurethane, High
Effects of polypropylene, polyvinyl chloride, polyethylene terephthalate, polyurethane, high- density polyethylene, and polystyrene microplastic on Nelumbo nucifera (Lotus) in water and sediment Maranda Esterhuizen ( maranda.esterhuizen@helsinki. ) University of Helsinki: Helsingin Yliopisto https://orcid.org/0000-0002-2342-3941 Youngjun Kim Korea Institute of Science and Technology Europe Forschungsgesellschaft mbH Research Article Keywords: Microplastics, oxidative stress, sediment, macrophyte, exposure, germination, seedling growth Posted Date: May 11th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-458889/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Loading [MathJax]/jax/output/CommonHTML/jax.js Page 1/20 Abstract Plastic waste is recognised as hazardous, with the risk increasing as the polymers break down in nature to secondary microplastics or even nanoplastics. The number of studies reporting on the prevalence of microplastic in every perceivable niche and bioavailable to biota is dramatically increasing. Knowledge of the ecotoxicology of microplastic is advancing as well; however, information regarding plants, specically aquatic macrophytes, is still lacking. The present study aimed to gain more information on the ecotoxicological effects of six different polymer types as 4 mm microplastic on the morphology (germination and growth) and the physiology (catalase and glutathione S-transferase activity) of the rooted aquatic macrophyte, Nelumbo nucifera. The role of sediment was also considered by conducting all exposure both in a sediment-containing and sediment-free exposure system. Polyvinyl chloride and polyurethane exposures caused the highest inhibition of germination and growth compared to the control. However, the presence of sediment signicantly decreased the adverse effects. -
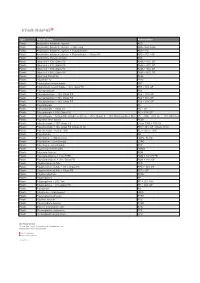
Type Material Name Abbreviation Plastic Acrylonitrile Butadiene
Type Material Name Abbreviation Plastic Acrylonitrile butadiene styrene ABS Plastic Acrylonitrile butadiene styrene - High-Temp ABS - high temp Plastic Acrylonitrile butadiene styrene + Polycarbonate ABS + PC Plastic Acrylonitrile butadiene styrene + Polycarbonate + Glass Fill ABS + PC + GF Plastic Acrylonitrile styrene acrylate ASA Plastic Nylon 6-6 + 10% Glass Fill PA66 + 10% GF Plastic Nylon 6-6 + 20% Glass Fill PA66 + 20% GF Plastic Nylon 6-6 + 30% Glass Fill PA66 + 30% GF Plastic Nylon 6-6 + 50% Glass Fill PA66 + 50% GF Plastic Nylon 6-6 Polyamide PA66 Plastic Polyamide 12 PA12 Plastic Polybutylene terephthalate PBT Plastic Polybutylene terephthalate + 30% Glass Fill PBT+ 30% GF Plastic Polycaprolactam PA6 Plastic Polycaprolactam + 20% Glass Fill PA6 + 20% GF Plastic Polycaprolactam + 30% Glass Fill PA6 + 30% GF Plastic Polycaprolactam + 50% Glass Fill PA6 + 50% GF Plastic Polycarbonate PC Plastic Polycarbonate + Glass Fill PC + GF Plastic Polycarbonate + 10% Glass Fill PC + 10% GF Plastic Polycarbonate + Acrylonitrile butadiene styrene + 20% Glass Fill + 10% Stainless Steel fiber PC + ABS + 20% GF + 10% SS Fiber Plastic Polyether ether ketone PEEK Plastic Polyetherimide + 30% Glass Fill Ultem 1000 + 30% GF Plastic Polyetherimide + 40% Glass Fill (Ultem 2410) PEI + 40% GF (Ultem 2410) Plastic Polyetherimide + Ultem 1000 PEI + Ultem 1000 Plastic Polyethylene PE Plastic Polyethylene - High-Density HDPE, PEHD Plastic Polyethylene - Low-Density LDPE Plastic Polyethylene terephthalate PET Plastic Polymethyl methacrylate PMMA Plastic Polyoxymethylene -

Environmental Impacts of Polyvinyl Chloride Building Materials
Environmental Impacts of Polyvinyl Chloride Building Materials by Joe Thornton, Ph.D. A Healthy Building Network Report Environmental Impacts of Polyvinyl Chloride Building Materials by Joe Thornton, Ph.D. A Healthy Building Network Report This report was prepared by the author and does not represent the opinions of The University of Oregon or any of its affiliates. © Healthy Buiding Network, 2002 Washington, D.C. The United States ISBN 0-9724632-0-8 Printed in the United States of America on acid-free, recycled paper, using soy-based ink. Photo Credits: p. 1 © Greenpeace p. 41 © Leuders/Greenpeace. p. 53 © Basel Action Network. PVC coated wire waste sorted and burned by villagers in Guiyu, China. Cancer causing polycyclic aro- matic hydrocarbons and dioxins will result from burning wires made from PVC and brominated flame retardants. p. 77 © Bryce lankard/Greenpeace 1996. Cows graze outside PVC manufacturing facility in Giesmar, Louisiana. Dioxin emissions to the environment move up the food chain. Fatty foods such as meat and dairy products are the primary source of dioxin exposure to most humans. p. 97 © Stone/Greenpeace 200. Lake Charles, Louisiana area children suffer health impacts due to pollution from PVC manufacturing facilities. Acknowledgements The Healthy Building acknowledges the following people and organiza- tions for their important contributions to this report. Dr. Joe Thornton’s willingness to accept this assignment on short notice and under a short deadline is testament to his ongoing commitment to the related goals -

Monomers – Styrene and Vinyl Chloride
Chemical Information Sheet Version 2.0 | March 2021 MONOMERS – STYRENE AND VINYL CHLORIDE Other Names Styrene: Ethenylbenzene, vinylbenzene, Monomers are chemical precursors that link together to phenylethene create polymer materials. Styrene and vinyl chloride are Vinyl Chloride: VCM, chloroethene monomers that may be present in low concentrations in some polymer materials. The presence of these monomers can be related to the process controls during CAS Number Substance polymer production. 100-42-5 Styrene Uses in the Supply Chain 75-01-4 Vinyl Chloride Styrene is a colorless liquid that evaporates easily which may be used to create polymers including polystyrene, ABS plastic, May Be Found In Styrene: Polystyrene, Acrylonitrile-butadiene- synthetic rubber (SBR) and other materials. Styrene can also be styrene (ABS) plastic, Styrene-butadiene rubber (SBR), styrene-divinylbenzene (S-DVB) used in plastic packaging and electrical parts. Vinyl Chloride: Polyvinyl chloride (PVC), Vinyl Chloride is used in production of polyvinyl chloride vinyl polymers, plastisol screen prints, plastic (PVC) and vinyl polymers, which can be hard or flexible parts, coatings for leather, synthetic leather and materials. PVC can be associated with plastisol screen prints, textiles plastic parts, and a variety of coatings on leather, synthetic leather, and textiles. Why Monomers Are Restricted ▪ Legislation in major markets globally restricts or regulates the presence of styrene and vinyl chloride in finished products or materials. ▪ Monomers can present a variety -

Interaction Between Plasticized Polyvinyl Chloride Waterproofing Membrane and Extruded Polystyrene Board, in the Inverted Flat Roof
MATERIALES DE CONSTRUCCIÓN Vol. 64, Issue 316, October–December 2014, e037 ISSN-L: 0465-2746 http://dx.doi.org/10.3989/mc.2014.008913 Interaction between plasticized polyvinyl chloride waterproofing membrane and extruded polystyrene board, in the inverted flat roof A. Pedrosa *, M. Del Río, C. Fonseca Universidad Politécnica de Madrid (Madrid, Spain) *[email protected] Received 22 October 2013 Accepted 6 June 2014 Available on line 2 December 2014 ABSTRACT: The inverted flat roof is a constructive system widely used in flat roof construction. In this con- structive solution, the insulation is placed over the waterproofing material as a protection. It is believed that this solution provides a longer life cycle; given the fact that it limits the thermal variation the waterproofing material bears up to the end of its life cycle. Consequently, the result will be providing a longer life to the waterproofing membrane. This constructive solution always incorporates polymers or other materials with a thermoplastic addition in their composition. Some polymers show interactions between them that can affect their integrity, and, at the same time, the bulk of the polymeric materials are incompatible. The extruded polystyrene board is always present in the inverted flat roof, and although it is an unbeatable product for this use, it presents incompatibilities and interactions with other materials, and these can affect their properties and therefore the durability of them. KEYWORDS: Inverted flat roof; Extruded polystyrene board; Waterproofing; Plasticizers migration; Single ply membrane Citation / Citar como: Pedrosa, A.; Del Río, M.; Fonseca, C. (2014) Interaction between plasticized polyvinyl chloride waterproofing membrane and extruded polystyrene board, in the inverted flat roof. -

Preparation and Characterization of an Asymmetric Porous
R ESEARCH ARTICLE ScienceAsia 34 (2008): 385–389 doi: 10.2306/scienceasia1513-1874.2008.34.385 Preparation and characterization of an asymmetric porous poly(vinyl chloride)/poly(methyl methacrylate-co- methacrylic acid) membrane Watchanida Chinpa Polymer Science Program, Faculty of Science, Prince of Songkla University, Hat Yai, Songkla 90112, Thailand e-mail: [email protected] Received 24 Oct 2007 Accepted 26 Aug 2008 ABSTRACT: Poly(vinyl chloride) (PVC) based membranes were prepared via a phase inversion method by casting a mixture solution containing PVC, poly(methyl methacrylate-co-methacrylic acid) (P(MMA-MAA)), and N-methyl pyrrolidone, using water as a coagulant. Measurement of the glass transition temperature showed that a PVC/P(MMA- MAA) system is virtually miscible. Cross-sections of the membranes showed that they were asymmetrical and had finger- like structures. Addition of P(MMA-MAA) to the PVC casting solution resulted in larger pores on the membrane surface, and the porosity and size of the finger-like pores increased as the content of P(MMA-MAA) increased. It was found that adding P(MMA-MAA) increased the porosity of the PVC membrane resulting in a higher permeation flux and hydrophilicity and lower rejection of bovine serum albumin, but a lower tensile strength and elongation at break. KEYWORDS: N-methyl pyrrolidone, polymer blends, hydrophilicity, phase inversion, finger-like structure INTRODUCTION However, the hydrophobicity of PVC causes heavy fouling on the membrane surface when a solution Phase inversion is the most commonly employed com- containing protein-like substances is treated. mercially available method for preparing microporous Blends of polymers are easy to prepare using polymeric membranes 1, 2. -

PVC (Polyvinyl Chloride)~ FACT SHEET & Talking Points
PVC (Polyvinyl Chloride)~ FACT SHEET & Talking Points http://archive.greenpeace.org/toxics/html/contenUpvc1.html http://www.greenpeace.org/usa/en/campaigns/toxics/go-pvc-free/ http://www.who.inUmediacentre/factsheets/fs225/en/ http://www.greenpeace.org/usa/en/campaigns/!oxics/go-pvc-free/ http://www.ens-newswire.com/ens/jan2011/2011-01-21-01.html What is PVC? >- The most environmentally damaging plastic. In fact, this commonplace plastic is one of the most toxic substances saturating our planet and its inhabitants >- From cradle to grave, the PVC Lifecycle (production, use and disposal) results in the release of toxic, chlorine based chemicals. P:. One of the world's largest dioxin sources ~ These toxins build up in water, air and in the food chain. ;... The results: severe health problems, including cancer, immune system damage and hormone disruption. ;... No one can escape contamination. P:. Everyone everywhere has measureable levels of chlorinated toxins in their bodies. How can we get rid of PVC? >- Since safer alternatives are available for virtually all uses of PVC, it is possible to protect human health and the environment by replacing and eventually phasing out this poison plastic. >- Policymakers at the local, state and federal level should enact and implement laws that steadily reduce impacts of PVC disposal and lead to a complete phase out of PVC use and waste incineration within ten years. >- A new materials policy for PVC that embraces aggressive source reduction of PVC should be adopted to steadily reduce the use of PVC over time liPage Background Information "Due to the omnipresence of dioxins, all people have background exposure and a certain level of dioxins in the body, leading to the so-called body burden. -

The Polyvinyl Chloride Debate: Why PVC Remains a Problematic Material
The polyvinyl chloride debate: Why PVC remains a problematic material Executive Summary In 2020, the European Commission commissioned a study entitled The use of PVC (Poly Vinyl Chloride) in the context of a non-toxic environment.1 It aims to identify and describe uncertainties (particularly from chemicals perspective), about PVC production, recovery, and end of life treatment and to help assess the role of PVC in the context of the European Green Deal and the Circular Economic Action Plan. The final report from this study is expected in November 2021. Within this paper we present a detailed insight into the complexity of health and environmental issues associated with the entire life cycle of PVC – including production, use, and disposal. The paper also includes examples of already successful phase-outs of PVC for specific applications. The information presented here should be considered in the on-going study of the Commission, collecting and updating factual data on the status of PVC in the EU economy. Ultimately, we hope it will support informed decisions of EU policymakers to support the achievement of a non-toxic environment in Europe. We note that the initial regulatory response to concerns about PVC focussed mainly on substitution and phase-out of phthalates, but failed to address wider health and environmental issues associated with PVC manufacture and use. While the impact of individual manufacturing plants can differ, global PVC industry impacts2 include: • Ozone depletion through the release of carbon tetrachloride • Contributions to climate change and environmental degradation from burning of fossil fuels to produce chlorine or coal-intensive acetylene-route PVC • Contaminating air and drinking water supplies • Substances harmful to human health and the environment: • Carcinogenic vinyl chloride monomer • Bioaccumulative toxics e.g. -

Number 1 Plastics – PET Or PETE (Polyethylene Terephthalate) Examples: Soda and Water Bottles; Mouthwash Bottles; Peanut Butt
Number 1 Plastics – PET or PETE (Polyethylene Terephthalate) Examples: Soda and water bottles; mouthwash bottles; peanut butter containers; salad dressing and vegetable oil containers; etc. Recycling: Plastic containers with a recycling symbol are collected in your blue recycling container. Recycled Into: Polar fleece fiber , tote bags, furniture, carpet, paneling, and occasionally made into new plastic containers. Number 2 Plastics – HDPE (High Density Polyethylene) Examples: Milk jugs, juice bottles; bleach, detergent and household cleaner bottles; shampoo bottles; some trash and shopping bags; motor oil bottles; cereal box liners; etc. Recycling: Plastic containers with a recycling symbol are collected in your blue recycling container, EXCEPT PLASTIC BAGS, please do not place plastic bags in your blue curbside recycling can. Recycled Into: Detergent bottles, recycling containers, floor tile, drainage pipe, benches, picnic tables, fencing Number 3 Plastics – PVC (Polyvinyl Chloride) Examples: Window cleaner and detergent bottles, shampoo bottles, clear food packaging, wire jacketing, medical equipment, siding, windows, piping; etc. Recycling: Few items are made from the material, but if you run across a CONTAINER with the #3 inside the recycling symbol it is collected in the blue curbside recycling can. Please do not put PVC pipe or other plastics not marked with the recy- cling symbol in your blue can. Recycled Into: Decks, paneling, mud flaps, roadway gutters, flooring, cables, speed bumps, mats Number 4 Plastics – LDPE (Low Density Polyethylene) Examples: Squeezable bottles; bread, dry cleaning and shopping bags; tote bags; carpet; etc. Recycling: Plastic containers with a recycling symbol are collected in your blue recycling container with the EXCEP- TION OF plastic bags.