And Poly(Vinyl Chloride) Nanoparticles with BHK-21 Cell Line
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2 Types of Noncrystalline Polymers
2 Types of Noncrystalline Polymers 1. Glassy polymer highly interpenetrated/entangled 2. Rubbery polymers random Gaussian coils Glass Transition Temperature εij Two viewpoints: • Increasing T: When kT > magnitude of εij, the thermal fluctuations can overcome local intermolecular bonds and the frozen (“glassy”) structure becomes “fluid-like”. • Decreasing T: As the temperature is lowered and T approaches Tg, the viscosity increases to ∞ and the material becomes “solid” Free Volume Theory of Tg Free volume, VF – extra space beyond what is present in an ordered crystalline packing (beyond the interstitial volume). VF(T) ≡ V(T) – V0(T) • V0 is occupied specific volume of atoms or molecules in the xline state and the spaces between them: ~ VXL. V(T) α • VF increases as T increases due to the Rate of cooling l difference in the thermal expansion V (T) αg XL coefficients (αg vs αl). FAST SLOW • V0(T) ≈ VXL(T) ↔ can take αg ≈αXL dV •V(T) = V (T ) + (T-T ) F T > T F F g g dT g Tg T • define fractional free volume, fF:Vf/V fF(T) = fF(Tg) + (T-Tg)αf αf = αl – αg Viewpoint: Tg occurs when available free volume drops below critical threshold for structural rearrangement [VITRIFICATION POINT], structure “jams up”. Tg Values of Amorphous Materials Table of representative amorphous solids, their bonding types, and their glass transition temperatures removed due to copyright restrictions. See Table 2.2 in Allen, S. M., and E.L. Thomas. The Structure of Materials. New York, NY: J. Wiley & Sons, 1999. Tg for Selected Polymers Glass Transition Temperature -

Blends of Polycarbonate Containing Fluorinated-Bisphenol-A and Polyvinyl Chloride
Europaisches Patentamt European Patent Office © Publication number: 0 576 057 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 93201533.2 int. Ci.5; C08L 69/00, C08L 27/06, C08G 64/10, //(C08L69/00, @ Date of filing: 28.05.93 27:06),(C08L27/06,69:00) © Priority: 01.06.92 US 891032 © Applicant: ENICHEM S.p.A. Piazza della Repubblica, 16 @ Date of publication of application: 1-20124 Milano(IT) 29.12.93 Bulletin 93/52 @ Inventor: Drzewinski, Michael A. © Designated Contracting States: 371 Clarksville Road, Princeton Junction AT BE CH DE DK ES FR GB GR IE IT LI LU MC New Jersey 08850(US) NL PT SE © Representative: Roggero, Sergio et al Ing. Barzano & Zanardo Milano S.p.A. Via Borgonuovo 10 1-20121 Milano (IT) © Blends of polycarbonate containing fluorinated-bisphenol-A and polyvinyl chloride. © Bisphenol A polycarbonate containing at least 15 mole % of 2,2-bis-(4-hydroxyphenyl)hexafluoropropane (6F-Bisphenol A) can be blended with polyvinyl chloride (PVC) to form a thermodynamically miscible, transpar- ent, single phase blend at all compositions. Such blends are flame resistant as well as resistant to attack by acids, bases and many organic solvents. CO Rank Xerox (UK) Business Services (3. 10/3.6/3.3. 1) EP 0 576 057 A1 BACKGROUND OF THE INVENTION Field of the Invention: 5 This invention pertains to mixtures of polyvinyl chloride (PVC) and polycarbonates which contain at least 15 mole % of fluorinated bisphenol monomer units (F-PC) such as 2,2-bis-(4-hydroxyphenyl)- hexafluoropropane (6F-bisphenol A), herein referred to as 6F-PC. -

United States Patent (19) 11 Patent Number: 4,481,333 Fleischer Et Al
United States Patent (19) 11 Patent Number: 4,481,333 Fleischer et al. 45 Date of Patent: Nov. 6, 1984 54 THERMOPLASTIC COMPOSITIONS 58 Field of Search ................................ 525/192, 199 COMPRISING WINYL CHLORIDE POLYMER, CLPE AND FLUOROPOLYMER 56) References Cited U.S. PATENT DOCUMENTS 75) Inventors: Dietrich Fleischer, Darmstadt; Eckhard Weber, Liederbach; 3,005,795 10/1961 Busse et al. ......................... 525/199 3,294,871 2/1966 Schmitt et al. ...... 52.5/154 X Johannes Brandrup, Wiesbaden, all 3,299,182 1/1967 Jennings et al. ... ... 525/192 of Fed. Rep. of Germany 3,334,157 8/1967 Larsen ..................... ... 525/99 73 Assignee: Hoechst Aktiengesellschaft, Fed. 3,940,456 2/1976 Fey et al. ............................ 525/192 Rep. of Germany Primary Examiner-Carman J. Seccuro (21) Appl. No.: 566,207 Attorney, Agent, or Firm-Connolly & Hutz 22 Filed: Dec. 28, 1983 57 ABSTRACT 30 Foreign Application Priority Data The invention relates to a thermoplastic composition which comprises vinyl chloride polymers and chlori Dec. 31, 1982 (DE Fed. Rep. of Germany ....... 3248.731 nated polyethylene and which contains finely divided 51) Int. Cl. ...................... C08L 23/28; C08L 27/06; fluoropolymers and has a markedly improved process C08L 27/18 ability, particularly when shaped by extrusion. 52 U.S. C. .................................... 525/192; 525/199; 525/239 7 Claims, No Drawings 4,481,333 2 iaries and do not provide a solution to the present prob THERMOPLASTC COMPOSITIONS lem. COMPRISINGVINYL CHLORIDE POLYMER, -
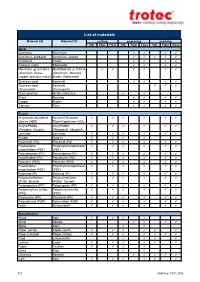
List of Materials
List of materials Material EN Material DE cutting engraving marking CO2 Fiber Flexx CO2 Fiber Flexx CO2 Fiber Flexx Metal Aluminum Aluminium Aluminum, anodized Aluminium, eloxiert Chromium Chrom Precious metal Edelmetall Metal foils up to 0.5mm Metallfolie bis zu 0,5mm (Aluminum, Brass, (Aluminium, Messing, Copper, precious metal) Kupfer, Edelmetall) Stainless steel Edelstahl Stainless steel Edelstahl (Thermark®) (Thermark®) Metal, painted Metall, lackiertes Brass Messing Copper Kupfer Titanium Titan Plastic Acrylonitrile butadiene Acrylnitril-Butadien- styrene (ABS) Styrol-Copolymer (ABS) Acrylic/PMMA Acryl/PMMA (Plexiglas, Altuglas, (Plexiglas®, Altuglas®, LaminatePerspex) LaminatePerspex®) Rubber Gummi Polyamide (PA) Polyamid (PA) Polybutylene Polybutylenterephthalat terephthalate (PBT) (PBT) Polycarbonate (PC) Polycarbonat (PC) Polyethylene (PE) Polyethylen (PE) Polyester (PES) Polyester (PES) Polyethylene Polyethylenterephthalat terephthalate (PET) (PET) Polyimide (PI) Polyimid (PI) Polyoxymethylene Polyoxymethylen (POM) -Delrin® (POM) - Delrin® Polypropylene (PP) Polypropylen (PP) Polyphenylene sulfide Polyphenylensulfid (PPS) (PPS) Polystyrene (PS) Polystyrol (PS) Polyurethane (PUR) Polyurethan (PUR) Foam Schaumstoff Miscellanious Wood Holz Mirror Spiegel Stone Stein Paper (white) Papier (weiß) Paper (colored) Papier (färbig) Food Lebensmittel Leather Leder -

Toxicity of the Pyrolysis and Combustion Products of Poly (Vinyl Chlorides): a Literature Assessment
FIRE AND MATERIALS VOL. II, 131-142 (1987) Toxicity of the Pyrolysis and Combustion Products of Poly (Vinyl Chlorides): A Literature Assessment Clayton Huggett and Barbara C. Levin* us Department of Commerce, National Bureau of Standards, National Engineering Laboratory, Center for Fire Research, Gaithersburg, MD 20899, USA Poly(vinyl chlorides) (PVC) constitute a major class of synthetic plastics. Many surveys of the voluminous literature have been performed. This report reviews the literature published in English from 1969 through 1984 and endeavors to be more interpretive than comprehensive. pve compounds, in general, are among the more fire resistant common organic polymers, natural or synthetic. The major products of thermal decomposition include hydrogen chloride, benzene and unsaturated hydrocarbons. In the presence of oxygen, carbon monoxide, carbon dioxide and water are included among the common combustion products. The main toxic products from PVC fires are hydrogen chloride (a sensory and pulmonary irritant) and carbon monoxide (an asphyxiant). The LCso values calculated for a series of natural and synthetic materials thermally decomposed according to the NBS toxicity test method ranged from 0.045 to 57 mgl-l in the flaming mode and from 0.045 to > 40mgl-l in the non-flaming mode. The LCso results for a PVC resin decomposed under the same conditions were 17 mg 1- 1 in the flaming mode and 20 mg 1- 1 in the non-flaming mode. These results indicate that PVC decomposition products are not extremely toxic when compared with those from other common building materials. When the combustion toxicity (based on their HCI content) of PVC materials is compared with pure HCI experiments, it appears that much of the post-exposure toxicity can be explained by the HCI tha t is genera ted. -

Use of a Crosslinking Agent, Crosslinked Polymer, and Uses Thereof
(19) TZZ Z_T (11) EP 2 452 970 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C08K 5/09 (2006.01) B32B 27/30 (2006.01) 21.01.2015 Bulletin 2015/04 C08L 29/04 (2006.01) C08L 101/00 (2006.01) G02B 5/30 (2006.01) C08K 5/098 (2006.01) (2006.01) (21) Application number: 12154871.3 C08K 5/10 (22) Date of filing: 29.08.2008 (54) Use of a crosslinking agent, crosslinked polymer, and uses thereof Verwendung eines Vernetzungsmittels, vernetztes Polymer und Verwendungen davon Utilisation d’un agent de réticulation, polymère réticulé et utilisations associées (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Kageyama, Hideki HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT Osaka-shi, Osaka 530-0018 (JP) RO SE SI SK TR • Tanaka, Shinichi Osaka-shi, Osaka 530-0018 (JP) (30) Priority: 31.08.2007 JP 2007225012 • Ono, Hiroyuki 18.03.2008 JP 2008069645 Osaka-shi, Osaka 530-0018 (JP) 04.04.2008 JP 2008098282 • Kuruma, Akiko 11.04.2008 JP 2008103494 Osaka-shi, Osaka 530-0018 (JP) 24.04.2008 JP 2008114149 24.04.2008 JP 2008114150 (74) Representative: Kuhnen & Wacker Patent- und Rechtsanwaltsbüro (43) Date of publication of application: Prinz-Ludwig-Straße 40A 16.05.2012 Bulletin 2012/20 85354 Freising (DE) (62) Document number(s) of the earlier application(s) in (56) References cited: accordance with Art. 76 EPC: WO-A1-2005/087711 JP-B2- 3 612 124 08828502.8 / 2 189 496 US-A1- 2004 102 586 (73) Proprietor: The Nippon Synthetic Chemical • DATABASE WPI Week 198020 Thomson Industry Co., Ltd. -

BASEL CONVENTION PLASTICS AMENDMENTS Institute of Scrap Recycling Industries (ISRI)
BASEL CONVENTION PLASTICS AMENDMENTS Institute of Scrap Recycling Industries (ISRI) Adina Renee Adler Assistant Vice President, International Affairs ©2019 Institute of Scrap Recycling Industries, Inc. All Rights Reserved The Driver 2 Basel Convention Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal Adopted 22 March 1989 Entered into force on 5 May 1992 Currently has 187 parties The United States is not a party. OCTOBER 2019 | ISRI.ORG 3 ©2019 ISRI, Inc. Basel Convention Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal Subjects hazardous wastes or other wastes with hazardous constituents / exhibiting hazardous characteristics to a “prior informed consent” (PIC) procedure before the material can be shipped across national borders. The PIC gives the receiving country the right of refusal if proper recycling or disposal operations do not exist. OCTOBER 2019 | ISRI.ORG 4 ©2019 ISRI, Inc. Basel Convention 14th Conference of the Parties (COP) held every two years to direct work on implementing the convention and addressing emerging issues. OCTOBER 2019 | ISRI.ORG 5 ©2019 ISRI, Inc. Norway’s Proposal Amend the following annexes so that trade controls are 1 put into place for certain plastics: Annex II: Non-hazardous, hard-to-recycle plastics Annex VIII: Hazardous plastics Annex IX: Non-hazardous, easy-to-recycle plastics (exempt from BC) Create a Partnership for Plastic Waste that would involve public and private stakeholders to find possible solutions 2 to plastic leakage. Terms of Reference negotiated and adopted. It will lead to non- binding initiatives to support developing country improvements to minimization, collection and proper handling of end-of-life plastics. -

Effects of Polypropylene, Polyvinyl Chloride, Polyethylene Terephthalate, Polyurethane, High
Effects of polypropylene, polyvinyl chloride, polyethylene terephthalate, polyurethane, high- density polyethylene, and polystyrene microplastic on Nelumbo nucifera (Lotus) in water and sediment Maranda Esterhuizen ( maranda.esterhuizen@helsinki. ) University of Helsinki: Helsingin Yliopisto https://orcid.org/0000-0002-2342-3941 Youngjun Kim Korea Institute of Science and Technology Europe Forschungsgesellschaft mbH Research Article Keywords: Microplastics, oxidative stress, sediment, macrophyte, exposure, germination, seedling growth Posted Date: May 11th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-458889/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Loading [MathJax]/jax/output/CommonHTML/jax.js Page 1/20 Abstract Plastic waste is recognised as hazardous, with the risk increasing as the polymers break down in nature to secondary microplastics or even nanoplastics. The number of studies reporting on the prevalence of microplastic in every perceivable niche and bioavailable to biota is dramatically increasing. Knowledge of the ecotoxicology of microplastic is advancing as well; however, information regarding plants, specically aquatic macrophytes, is still lacking. The present study aimed to gain more information on the ecotoxicological effects of six different polymer types as 4 mm microplastic on the morphology (germination and growth) and the physiology (catalase and glutathione S-transferase activity) of the rooted aquatic macrophyte, Nelumbo nucifera. The role of sediment was also considered by conducting all exposure both in a sediment-containing and sediment-free exposure system. Polyvinyl chloride and polyurethane exposures caused the highest inhibition of germination and growth compared to the control. However, the presence of sediment signicantly decreased the adverse effects. -

Miscibility Studies, Characterization and Properties Evaluation of Pvc/Pmma Blends
International Journal of Scientific & Engineering Research, Volume 7, Issue 8, August-2016 1766 ISSN 2229-5518 MISCIBILITY STUDIES, CHARACTERIZATION AND PROPERTIES EVALUATION OF PVC/PMMA BLENDS. Chandramohan, B.Rajam, V.V.Basava Rao Abstract: Poly Vinyl Chloride (PVC) and Poly methyl methacrylate (PMMA) blends are prepared by injection moulding method over an entire composition range of 0/100, 25/75, 50/50, 75/25 and 100/0 by weight. The miscibility of the blends was characterized by dynamic mechanical thermal analysis. The effect of polymer-polymer interactions on the miscibility of PVC/PMMA blends were studied using the method. The effect of blending poly (methyl methacrylate) in various proportions with suitably stabilized and plasticized poly (vinyl chloride) was studied with reference to their physical, mechanical, and thermal properties. Distribution of the phases in the blends will be studied through scanning electron microscopy. These properties of PVC/PMMA blends are correlated with spectroscopic investigation. Keywords: Poly Vinyl Chloride, Poly Methyl Methacrylate, dynamic mechanical analysis, Morphology studies, Injection moulding —————————— —————————— 1.0 Introduction: Poly vinyl chloride (PVC) is a normal impact, high corrosion resistant thermoplastic. Due to the exceptional corrosion resistance of the material, it is used where maximum chemical resistance is necessary. The low cost is also one favourable factor in choosing the compound for various applications in industries and in domestic usage. Approximately 75% of the world’s PVC is produced by the suspension polymerization (S- PVC) process inIJSER which the polymerization is carried out inside vinyl chloride monomer (VCM) droplets dispersed in water. The product is in the form of porous 100-150 µm- diameter grains. -

The Nuclear Membrane Determines the Timing of DNA Replication in Xenopus Egg Extracts Gregory H
The Nuclear Membrane Determines the Timing of DNA Replication in Xenopus Egg Extracts Gregory H. Leno and Ronald A. Laskey Cancer Research Campaign Molecular Embryology Group, Department of Zoology, University of Cambridge, Cambridge CB2 3EJ, England Abstract. We have exploited a property of chicken scribed in cultured cells. In contrast to the asynchro- erythrocyte nuclei to analyze the regulation of DNA nous replication seen :between individual nuclei, repli- replication in a cell-free system from Xenopus eggs. cation within multinuclear aggregates was syn- Many individual demembranated nuclei added to the chronous. There ;was a uniform distribution and extract often became enclosed within a common nu- similar fluorescent intensity of the replication loci clear membrane. Nuclei within such a "multinuclear throughout all the nuclei enclosed within the same aggregate" lacked individual membranes but shared the membrane. However, different multinuclear aggregates perimeter membrane of the aggregate. Individual replicated out of synchrony with each other indicating nuclei that were excluded from the aggregates initiated that each membrane-bound aggregate acts as an in- DNA synthesis at different times over a 10-12-h dividual unit of replication. These data indicate that period, as judged by incorporation of biotinylated the nuclear membrane defines the unit of DNA repli- dUTP into discrete replication foci at early times, fol- cation and determines the timing of DNA synthesis in lowed by uniformly intense incorporation at later egg extract resulting in highly coordinated triggering times. Replication forks were clustered in spots, rings, of DNA replication on the DNA it encloses. and horsesh0e-shaped structures similar to those de- NITIATION of DNA replication occurs at thousands of defines the unit of DNA replication in the egg extract. -

Polyvinylbutyral HVB-0001
Technical Information Polyvinylbutyral HVB-0001 Description: HVB-0001 is high viscosity thermoplastic Polyvinylbutyral Resin combining a very large surface area with medium molecular weight. Its sensorial properties are outstanding. The product is soluble in solvents like Alcohols or Glycol Ethers, it is compatible with Esters and Ketones. HVB-0001 shows excellent compatibility with other resins like Nitrocellulose, Epoxy Resins, Epoxy Alkyds, Phenolic Resins, Polyurethanes, Melamine-Formaldehyde Resins, Ketonic Resins and Polyamides. With its free hydroxyl groups the material is chemically reactive. Application: Printing Inks and lacquers: HVB-0001 is an ideal choice for formation of printing inks for flexible packaging (Flexographic & Gravure application). The product offers good water resistance combined with good blocking resistance, excellent pigment wetting and good flow. It shows good adhesion to substrates like Cellulose Acetate, Polyester, Polystyrene or Metalized Films. HVB-0001 is suitable for food packaging applications. Wash Primers: HVB-0001 can be used for formulation of wash primers for steel storage tanks, bottom of ships, airplane, bridges, dam locks, trailers, farm equipment, automotive, rail coaches etc. The material offers flexible films with good adhesion to metal surfaces. Adhesives: HVB-0001 acts as a plasticizer for adhesive formulations based on Epoxy, Phenolic, Melamine-Formaldehyde Resins and Isocyanates. It helps improving interfacial adhesion, flexibility, impact strength and resistance against solvent, chemicals or high temperature. Ceramics: HVB-0001 solution can be used as a temporary binder for ceramic powder. Typically 2 – 4 % PVB-resin are added to Ceramic. During sintering process HVB-0001 undergoes thermal degradation leaving virtually no residue. It leads to very good green strength and dimensional stability. -
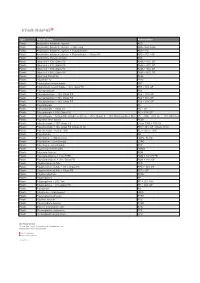
Type Material Name Abbreviation Plastic Acrylonitrile Butadiene
Type Material Name Abbreviation Plastic Acrylonitrile butadiene styrene ABS Plastic Acrylonitrile butadiene styrene - High-Temp ABS - high temp Plastic Acrylonitrile butadiene styrene + Polycarbonate ABS + PC Plastic Acrylonitrile butadiene styrene + Polycarbonate + Glass Fill ABS + PC + GF Plastic Acrylonitrile styrene acrylate ASA Plastic Nylon 6-6 + 10% Glass Fill PA66 + 10% GF Plastic Nylon 6-6 + 20% Glass Fill PA66 + 20% GF Plastic Nylon 6-6 + 30% Glass Fill PA66 + 30% GF Plastic Nylon 6-6 + 50% Glass Fill PA66 + 50% GF Plastic Nylon 6-6 Polyamide PA66 Plastic Polyamide 12 PA12 Plastic Polybutylene terephthalate PBT Plastic Polybutylene terephthalate + 30% Glass Fill PBT+ 30% GF Plastic Polycaprolactam PA6 Plastic Polycaprolactam + 20% Glass Fill PA6 + 20% GF Plastic Polycaprolactam + 30% Glass Fill PA6 + 30% GF Plastic Polycaprolactam + 50% Glass Fill PA6 + 50% GF Plastic Polycarbonate PC Plastic Polycarbonate + Glass Fill PC + GF Plastic Polycarbonate + 10% Glass Fill PC + 10% GF Plastic Polycarbonate + Acrylonitrile butadiene styrene + 20% Glass Fill + 10% Stainless Steel fiber PC + ABS + 20% GF + 10% SS Fiber Plastic Polyether ether ketone PEEK Plastic Polyetherimide + 30% Glass Fill Ultem 1000 + 30% GF Plastic Polyetherimide + 40% Glass Fill (Ultem 2410) PEI + 40% GF (Ultem 2410) Plastic Polyetherimide + Ultem 1000 PEI + Ultem 1000 Plastic Polyethylene PE Plastic Polyethylene - High-Density HDPE, PEHD Plastic Polyethylene - Low-Density LDPE Plastic Polyethylene terephthalate PET Plastic Polymethyl methacrylate PMMA Plastic Polyoxymethylene