Abbreviations for Various Plastic Materials Typical Molding Problems and Possible Causes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2 Types of Noncrystalline Polymers
2 Types of Noncrystalline Polymers 1. Glassy polymer highly interpenetrated/entangled 2. Rubbery polymers random Gaussian coils Glass Transition Temperature εij Two viewpoints: • Increasing T: When kT > magnitude of εij, the thermal fluctuations can overcome local intermolecular bonds and the frozen (“glassy”) structure becomes “fluid-like”. • Decreasing T: As the temperature is lowered and T approaches Tg, the viscosity increases to ∞ and the material becomes “solid” Free Volume Theory of Tg Free volume, VF – extra space beyond what is present in an ordered crystalline packing (beyond the interstitial volume). VF(T) ≡ V(T) – V0(T) • V0 is occupied specific volume of atoms or molecules in the xline state and the spaces between them: ~ VXL. V(T) α • VF increases as T increases due to the Rate of cooling l difference in the thermal expansion V (T) αg XL coefficients (αg vs αl). FAST SLOW • V0(T) ≈ VXL(T) ↔ can take αg ≈αXL dV •V(T) = V (T ) + (T-T ) F T > T F F g g dT g Tg T • define fractional free volume, fF:Vf/V fF(T) = fF(Tg) + (T-Tg)αf αf = αl – αg Viewpoint: Tg occurs when available free volume drops below critical threshold for structural rearrangement [VITRIFICATION POINT], structure “jams up”. Tg Values of Amorphous Materials Table of representative amorphous solids, their bonding types, and their glass transition temperatures removed due to copyright restrictions. See Table 2.2 in Allen, S. M., and E.L. Thomas. The Structure of Materials. New York, NY: J. Wiley & Sons, 1999. Tg for Selected Polymers Glass Transition Temperature -

Improving Plastics Management: Trends, Policy Responses, and the Role of International Co-Operation and Trade
Improving Plastics Management: Trends, policy responses, and the role of international co-operation and trade POLICY PERSPECTIVES OECD ENVIRONMENT POLICY PAPER NO. 12 OECD . 3 This Policy Paper comprises the Background Report prepared by the OECD for the G7 Environment, Energy and Oceans Ministers. It provides an overview of current plastics production and use, the environmental impacts that this is generating and identifies the reasons for currently low plastics recycling rates, as well as what can be done about it. Disclaimers This paper is published under the responsibility of the Secretary-General of the OECD. The opinions expressed and the arguments employed herein do not necessarily reflect the official views of OECD member countries. This document and any map included herein are without prejudice to the status of or sovereignty over any territory, to the delimitation of international frontiers and boundaries and to the name of any territory, city or area. For Israel, change is measured between 1997-99 and 2009-11. The statistical data for Israel are supplied by and under the responsibility of the relevant Israeli authorities. The use of such data by the OECD is without prejudice to the status of the Golan Heights, East Jerusalem and Israeli settlements in the West Bank under the terms of international law. Copyright You can copy, download or print OECD content for your own use, and you can include excerpts from OECD publications, databases and multimedia products in your own documents, presentations, blogs, websites and teaching materials, provided that suitable acknowledgment of OECD as source and copyright owner is given. -
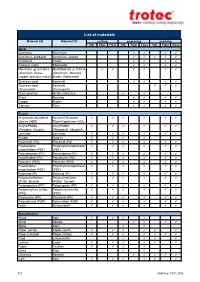
List of Materials
List of materials Material EN Material DE cutting engraving marking CO2 Fiber Flexx CO2 Fiber Flexx CO2 Fiber Flexx Metal Aluminum Aluminium Aluminum, anodized Aluminium, eloxiert Chromium Chrom Precious metal Edelmetall Metal foils up to 0.5mm Metallfolie bis zu 0,5mm (Aluminum, Brass, (Aluminium, Messing, Copper, precious metal) Kupfer, Edelmetall) Stainless steel Edelstahl Stainless steel Edelstahl (Thermark®) (Thermark®) Metal, painted Metall, lackiertes Brass Messing Copper Kupfer Titanium Titan Plastic Acrylonitrile butadiene Acrylnitril-Butadien- styrene (ABS) Styrol-Copolymer (ABS) Acrylic/PMMA Acryl/PMMA (Plexiglas, Altuglas, (Plexiglas®, Altuglas®, LaminatePerspex) LaminatePerspex®) Rubber Gummi Polyamide (PA) Polyamid (PA) Polybutylene Polybutylenterephthalat terephthalate (PBT) (PBT) Polycarbonate (PC) Polycarbonat (PC) Polyethylene (PE) Polyethylen (PE) Polyester (PES) Polyester (PES) Polyethylene Polyethylenterephthalat terephthalate (PET) (PET) Polyimide (PI) Polyimid (PI) Polyoxymethylene Polyoxymethylen (POM) -Delrin® (POM) - Delrin® Polypropylene (PP) Polypropylen (PP) Polyphenylene sulfide Polyphenylensulfid (PPS) (PPS) Polystyrene (PS) Polystyrol (PS) Polyurethane (PUR) Polyurethan (PUR) Foam Schaumstoff Miscellanious Wood Holz Mirror Spiegel Stone Stein Paper (white) Papier (weiß) Paper (colored) Papier (färbig) Food Lebensmittel Leather Leder -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

TSCA Inventory Representation for Products Containing Two Or More
mixtures.txt TOXIC SUBSTANCES CONTROL ACT INVENTORY REPRESENTATION FOR PRODUCTS CONTAINING TWO OR MORE SUBSTANCES: FORMULATED AND STATUTORY MIXTURES I. Introduction This paper explains the conventions that are applied to listings of certain mixtures for the Chemical Substance Inventory that is maintained by the U.S. Environmental Protection Agency (EPA) under the Toxic Substances Control Act (TSCA). This paper discusses the Inventory representation of mixtures of substances that do not react together (i.e., formulated mixtures) as well as those combinations that are formed during certain manufacturing activities and are designated as mixtures by the Agency (i.e., statutory mixtures). Complex reaction products are covered in a separate paper. The Agency's goal in developing this paper is to make it easier for the users of the Inventory to interpret Inventory listings and to understand how new mixtures would be identified for Inventory inclusion. Fundamental to the Inventory as a whole is the principle that entries on the Inventory are identified as precisely as possible for the commercial chemical substance, as reported by the submitter. Substances that are chemically indistinguishable, or even identical, may be listed differently on the Inventory, depending on the degree of knowledge that the submitters possess and report about such substances, as well as how submitters intend to represent the chemical identities to the Agency and to customers. Although these chemically indistinguishable substances are named differently on the Inventory, this is not a "nomenclature" issue, but an issue of substance representation. Submitters should be aware that their choice for substance representation plays an important role in the Agency's determination of how the substance will be listed on the Inventory. -

Chapter 2 the Solid Materials of the Earth's Surface
CHAPTER 2 THE SOLID MATERIALS OF THE EARTH’S SURFACE 1. INTRODUCTION 1.1 To a great extent in this course, we will be dealing with processes that act on the solid materials at and near the Earth’s surface. This chapter might better be called “the ground beneath your feet”. This is the place to deal with the nature of the Earth’s surface materials, which in later sections of the chapter I will be calling regolith, sediment, and soil. 1.2 I purposely did not specify any previous knowledge of geology as a prerequisite for this course, so it is important, here in the first part of this chapter, for me to provide you with some background on Earth materials. 1.3 We will be dealing almost exclusively with the Earth’s continental surfaces. There are profound geological differences between the continents and the ocean basins, in terms of origin, age, history, and composition. Here I’ll present, very briefly, some basic things about geology. (For more depth on such matters you would need to take a course like “The Earth: What It Is, How It Works”, given in the Harvard Extension program in the fall semester of 2005– 2006 and likely to be offered again in the not-too-distant future.) 1.4 In a gross sense, the Earth is a layered body (Figure 2-1). To a first approximation, it consists of concentric shells: the core, the mantle, and the crust. Figure 2-1: Schematic cross section through the Earth. 73 The core: The core consists mostly of iron, alloyed with a small percentage of certain other chemical elements. -

Use of a Crosslinking Agent, Crosslinked Polymer, and Uses Thereof
(19) TZZ Z_T (11) EP 2 452 970 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C08K 5/09 (2006.01) B32B 27/30 (2006.01) 21.01.2015 Bulletin 2015/04 C08L 29/04 (2006.01) C08L 101/00 (2006.01) G02B 5/30 (2006.01) C08K 5/098 (2006.01) (2006.01) (21) Application number: 12154871.3 C08K 5/10 (22) Date of filing: 29.08.2008 (54) Use of a crosslinking agent, crosslinked polymer, and uses thereof Verwendung eines Vernetzungsmittels, vernetztes Polymer und Verwendungen davon Utilisation d’un agent de réticulation, polymère réticulé et utilisations associées (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Kageyama, Hideki HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT Osaka-shi, Osaka 530-0018 (JP) RO SE SI SK TR • Tanaka, Shinichi Osaka-shi, Osaka 530-0018 (JP) (30) Priority: 31.08.2007 JP 2007225012 • Ono, Hiroyuki 18.03.2008 JP 2008069645 Osaka-shi, Osaka 530-0018 (JP) 04.04.2008 JP 2008098282 • Kuruma, Akiko 11.04.2008 JP 2008103494 Osaka-shi, Osaka 530-0018 (JP) 24.04.2008 JP 2008114149 24.04.2008 JP 2008114150 (74) Representative: Kuhnen & Wacker Patent- und Rechtsanwaltsbüro (43) Date of publication of application: Prinz-Ludwig-Straße 40A 16.05.2012 Bulletin 2012/20 85354 Freising (DE) (62) Document number(s) of the earlier application(s) in (56) References cited: accordance with Art. 76 EPC: WO-A1-2005/087711 JP-B2- 3 612 124 08828502.8 / 2 189 496 US-A1- 2004 102 586 (73) Proprietor: The Nippon Synthetic Chemical • DATABASE WPI Week 198020 Thomson Industry Co., Ltd. -

Review of Thermal Properties of Graphene and Few-Layer
Zhong Yan, Denis L. Nika and Alexander A. Balandin (invited review, submitted in 2014) INVITED REVIEW PAPER Review of Thermal Properties of Graphene and Few-Layer Graphene: Applications in Electronics Zhong Yan1, Denis L. Nika1,2 and Alexander A. Balandin1,3 1Nano-Device Laboratory, Department of Electrical Engineering and Materials Science and Engineering Program, Bourns College of Engineering, University of California – Riverside, Riverside, California, 92521 USA 2E. Pokatilov Laboratory of Physics and Engineering of Nanomaterials, Department of Physics and Engineering, Moldova State University, Chisinau, MD-2009, Republic of Moldova 3Quantum Seed LLC, 1190 Columbia Avenue, Riverside, California 92507 USA Abstract We review thermal properties of graphene and few-layer graphene, and discuss applications of these materials in thermal management of advanced electronics. The intrinsic thermal conductivity of graphene – among the highest of known materials – is dominated by phonons near the room temperature. The examples of thermal management applications include the few-layer graphene heat spreaders integrated near the heat generating areas of the high-power density transistors. It has been demonstrated that few-layer graphene heat spreaders can lower the hot-spot temperature during device operation resulting in improved performance and reliability of the devices. Keywords: graphene, thermal conductivity, heat spreaders, thermal management 1 Zhong Yan, Denis L. Nika and Alexander A. Balandin (invited review, submitted in 2014) I. Introduction Thermal management represents a major challenge in the state-of-the-art electronics due to rapid increase of power densities [1, 2]. Efficient heat removal has become a critical issue for the performance and reliability of modern electronic, optoelectronic, photonic devices and systems. -

BASEL CONVENTION PLASTICS AMENDMENTS Institute of Scrap Recycling Industries (ISRI)
BASEL CONVENTION PLASTICS AMENDMENTS Institute of Scrap Recycling Industries (ISRI) Adina Renee Adler Assistant Vice President, International Affairs ©2019 Institute of Scrap Recycling Industries, Inc. All Rights Reserved The Driver 2 Basel Convention Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal Adopted 22 March 1989 Entered into force on 5 May 1992 Currently has 187 parties The United States is not a party. OCTOBER 2019 | ISRI.ORG 3 ©2019 ISRI, Inc. Basel Convention Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal Subjects hazardous wastes or other wastes with hazardous constituents / exhibiting hazardous characteristics to a “prior informed consent” (PIC) procedure before the material can be shipped across national borders. The PIC gives the receiving country the right of refusal if proper recycling or disposal operations do not exist. OCTOBER 2019 | ISRI.ORG 4 ©2019 ISRI, Inc. Basel Convention 14th Conference of the Parties (COP) held every two years to direct work on implementing the convention and addressing emerging issues. OCTOBER 2019 | ISRI.ORG 5 ©2019 ISRI, Inc. Norway’s Proposal Amend the following annexes so that trade controls are 1 put into place for certain plastics: Annex II: Non-hazardous, hard-to-recycle plastics Annex VIII: Hazardous plastics Annex IX: Non-hazardous, easy-to-recycle plastics (exempt from BC) Create a Partnership for Plastic Waste that would involve public and private stakeholders to find possible solutions 2 to plastic leakage. Terms of Reference negotiated and adopted. It will lead to non- binding initiatives to support developing country improvements to minimization, collection and proper handling of end-of-life plastics. -

Introduction to Material Science and Engineering Presentation.(Pdf)
Introduction to Material Science and Engineering Introduction What is material science? Definition 1: A branch of science that focuses on materials; interdisciplinary field composed of physics and chemistry. Definition 2: Relationship of material properties to its composition and structure. What is a material scientist? A person who uses his/her combined knowledge of physics, chemistry and metallurgy to exploit property-structure combinations for practical use. What are materials? What do we mean when we say “materials”? 1. Metals 2. Ceramics 3. Polymers 4. Composites - aluminum - clay - polyvinyl chloride (PVC) - wood - copper - silica glass - Teflon - carbon fiber resins - steel (iron alloy) - alumina - various plastics - concrete - nickel - quartz - glue (adhesives) - titanium - Kevlar semiconductors (computer chips, etc.) = ceramics, composites nanomaterials = ceramics, metals, polymers, composites Length Scales of Material Science • Atomic – < 10-10 m • Nano – 10-9 m • Micro – 10-6 m • Macro – > 10-3 m Atomic Structure – 10-10 m • Pertains to atom electron structure and atomic arrangement • Atom length scale – Includes electron structure – atomic bonding • ionic • covalent • metallic • London dispersion forces (Van der Waals) – Atomic ordering – long range (metals), short range (glass) • 7 lattices – cubic, hexagonal among most prevalent for engineering metals and ceramics • Different packed structures include: Gives total of 14 different crystalline arrangements (Bravais Lattices). – Primitive, body-centered, face-centered Nano Structure – 10-9 m • Length scale that pertains to clusters of atoms that make up small particles or material features • Show interesting properties because increase surface area to volume ratio – More atoms on surface compared to bulk atoms – Optical, magnetic, mechanical and electrical properties change Microstructure – 10-6 • Larger features composed of either nanostructured materials or periodic arrangements of atoms known as crystals • Features are visible with high magnification in light microscope. -

Polyvinylbutyral HVB-0001
Technical Information Polyvinylbutyral HVB-0001 Description: HVB-0001 is high viscosity thermoplastic Polyvinylbutyral Resin combining a very large surface area with medium molecular weight. Its sensorial properties are outstanding. The product is soluble in solvents like Alcohols or Glycol Ethers, it is compatible with Esters and Ketones. HVB-0001 shows excellent compatibility with other resins like Nitrocellulose, Epoxy Resins, Epoxy Alkyds, Phenolic Resins, Polyurethanes, Melamine-Formaldehyde Resins, Ketonic Resins and Polyamides. With its free hydroxyl groups the material is chemically reactive. Application: Printing Inks and lacquers: HVB-0001 is an ideal choice for formation of printing inks for flexible packaging (Flexographic & Gravure application). The product offers good water resistance combined with good blocking resistance, excellent pigment wetting and good flow. It shows good adhesion to substrates like Cellulose Acetate, Polyester, Polystyrene or Metalized Films. HVB-0001 is suitable for food packaging applications. Wash Primers: HVB-0001 can be used for formulation of wash primers for steel storage tanks, bottom of ships, airplane, bridges, dam locks, trailers, farm equipment, automotive, rail coaches etc. The material offers flexible films with good adhesion to metal surfaces. Adhesives: HVB-0001 acts as a plasticizer for adhesive formulations based on Epoxy, Phenolic, Melamine-Formaldehyde Resins and Isocyanates. It helps improving interfacial adhesion, flexibility, impact strength and resistance against solvent, chemicals or high temperature. Ceramics: HVB-0001 solution can be used as a temporary binder for ceramic powder. Typically 2 – 4 % PVB-resin are added to Ceramic. During sintering process HVB-0001 undergoes thermal degradation leaving virtually no residue. It leads to very good green strength and dimensional stability. -

Phonons in Graphene and Van Der Waals Materials
Phonons in Graphene and van der Waals Materials Alexander A. Balandin Department of Electrical Engineering and Materials Science and Engineering Program University of California – Riverside MRS Medal Talk Fall 2013 Nano-Device Laboratory (NDL) Department of Electrical Engineering University of California – Riverside Profile: experimental and theoretical research in advanced materials and nano-devices Nanoscale Characterization PI: Alexander A. Balandin Phononics Research Thermal and Electrical & Applications Characterization Electronic Devices and Circuits Theory and Modeling Direct Energy Conversion Device Design and Raman, Fluorescence Characterization and PL Spectroscopy Optoelectronics Bio- Nanotech Alexander A. Balandin, University of California - Riverside Outline Introduction and Basic Definitions Phonons Thermal conductivity Thermal Conductivity of Graphene Raman spectroscopy Optothermal technique Theoretical interpretation Graphene Thermal Applications TIMs and PCMs Heat spreaders Phonons in van der Waals Materials Raman metrology Charge-density waves Outlook: Phononics and Phonon Engineering 3 Alexander A. Balandin, University of California - Riverside Practical Motivations: Why Material Scientists Should Study Thermal Properties? Data is after R. Mahajan et al., Proceed. IEEE (2006) No BIG fan solutions! The switch to multi-core designs alleviates the growth in the thermal design power (TDP) IEEE Spectrum illustration of the increase but does not solve the thermal issues in the feature hot-spot problem article Chill Out: New Materials and Designs Can Keep Chips Non-uniform power densities Cool by A.A. Balandin. leading to hot-spots (>500 W/cm2) Alexander A. Balandin, University of California - Riverside Basics of Phonons The quantum of the energy of a lattice vibration is called a phonon in analogy with the photon of the electromagnetic wave.