Review of Thermal Properties of Graphene and Few-Layer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Improving Plastics Management: Trends, Policy Responses, and the Role of International Co-Operation and Trade
Improving Plastics Management: Trends, policy responses, and the role of international co-operation and trade POLICY PERSPECTIVES OECD ENVIRONMENT POLICY PAPER NO. 12 OECD . 3 This Policy Paper comprises the Background Report prepared by the OECD for the G7 Environment, Energy and Oceans Ministers. It provides an overview of current plastics production and use, the environmental impacts that this is generating and identifies the reasons for currently low plastics recycling rates, as well as what can be done about it. Disclaimers This paper is published under the responsibility of the Secretary-General of the OECD. The opinions expressed and the arguments employed herein do not necessarily reflect the official views of OECD member countries. This document and any map included herein are without prejudice to the status of or sovereignty over any territory, to the delimitation of international frontiers and boundaries and to the name of any territory, city or area. For Israel, change is measured between 1997-99 and 2009-11. The statistical data for Israel are supplied by and under the responsibility of the relevant Israeli authorities. The use of such data by the OECD is without prejudice to the status of the Golan Heights, East Jerusalem and Israeli settlements in the West Bank under the terms of international law. Copyright You can copy, download or print OECD content for your own use, and you can include excerpts from OECD publications, databases and multimedia products in your own documents, presentations, blogs, websites and teaching materials, provided that suitable acknowledgment of OECD as source and copyright owner is given. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

TSCA Inventory Representation for Products Containing Two Or More
mixtures.txt TOXIC SUBSTANCES CONTROL ACT INVENTORY REPRESENTATION FOR PRODUCTS CONTAINING TWO OR MORE SUBSTANCES: FORMULATED AND STATUTORY MIXTURES I. Introduction This paper explains the conventions that are applied to listings of certain mixtures for the Chemical Substance Inventory that is maintained by the U.S. Environmental Protection Agency (EPA) under the Toxic Substances Control Act (TSCA). This paper discusses the Inventory representation of mixtures of substances that do not react together (i.e., formulated mixtures) as well as those combinations that are formed during certain manufacturing activities and are designated as mixtures by the Agency (i.e., statutory mixtures). Complex reaction products are covered in a separate paper. The Agency's goal in developing this paper is to make it easier for the users of the Inventory to interpret Inventory listings and to understand how new mixtures would be identified for Inventory inclusion. Fundamental to the Inventory as a whole is the principle that entries on the Inventory are identified as precisely as possible for the commercial chemical substance, as reported by the submitter. Substances that are chemically indistinguishable, or even identical, may be listed differently on the Inventory, depending on the degree of knowledge that the submitters possess and report about such substances, as well as how submitters intend to represent the chemical identities to the Agency and to customers. Although these chemically indistinguishable substances are named differently on the Inventory, this is not a "nomenclature" issue, but an issue of substance representation. Submitters should be aware that their choice for substance representation plays an important role in the Agency's determination of how the substance will be listed on the Inventory. -

Chapter 2 the Solid Materials of the Earth's Surface
CHAPTER 2 THE SOLID MATERIALS OF THE EARTH’S SURFACE 1. INTRODUCTION 1.1 To a great extent in this course, we will be dealing with processes that act on the solid materials at and near the Earth’s surface. This chapter might better be called “the ground beneath your feet”. This is the place to deal with the nature of the Earth’s surface materials, which in later sections of the chapter I will be calling regolith, sediment, and soil. 1.2 I purposely did not specify any previous knowledge of geology as a prerequisite for this course, so it is important, here in the first part of this chapter, for me to provide you with some background on Earth materials. 1.3 We will be dealing almost exclusively with the Earth’s continental surfaces. There are profound geological differences between the continents and the ocean basins, in terms of origin, age, history, and composition. Here I’ll present, very briefly, some basic things about geology. (For more depth on such matters you would need to take a course like “The Earth: What It Is, How It Works”, given in the Harvard Extension program in the fall semester of 2005– 2006 and likely to be offered again in the not-too-distant future.) 1.4 In a gross sense, the Earth is a layered body (Figure 2-1). To a first approximation, it consists of concentric shells: the core, the mantle, and the crust. Figure 2-1: Schematic cross section through the Earth. 73 The core: The core consists mostly of iron, alloyed with a small percentage of certain other chemical elements. -

Introduction to Material Science and Engineering Presentation.(Pdf)
Introduction to Material Science and Engineering Introduction What is material science? Definition 1: A branch of science that focuses on materials; interdisciplinary field composed of physics and chemistry. Definition 2: Relationship of material properties to its composition and structure. What is a material scientist? A person who uses his/her combined knowledge of physics, chemistry and metallurgy to exploit property-structure combinations for practical use. What are materials? What do we mean when we say “materials”? 1. Metals 2. Ceramics 3. Polymers 4. Composites - aluminum - clay - polyvinyl chloride (PVC) - wood - copper - silica glass - Teflon - carbon fiber resins - steel (iron alloy) - alumina - various plastics - concrete - nickel - quartz - glue (adhesives) - titanium - Kevlar semiconductors (computer chips, etc.) = ceramics, composites nanomaterials = ceramics, metals, polymers, composites Length Scales of Material Science • Atomic – < 10-10 m • Nano – 10-9 m • Micro – 10-6 m • Macro – > 10-3 m Atomic Structure – 10-10 m • Pertains to atom electron structure and atomic arrangement • Atom length scale – Includes electron structure – atomic bonding • ionic • covalent • metallic • London dispersion forces (Van der Waals) – Atomic ordering – long range (metals), short range (glass) • 7 lattices – cubic, hexagonal among most prevalent for engineering metals and ceramics • Different packed structures include: Gives total of 14 different crystalline arrangements (Bravais Lattices). – Primitive, body-centered, face-centered Nano Structure – 10-9 m • Length scale that pertains to clusters of atoms that make up small particles or material features • Show interesting properties because increase surface area to volume ratio – More atoms on surface compared to bulk atoms – Optical, magnetic, mechanical and electrical properties change Microstructure – 10-6 • Larger features composed of either nanostructured materials or periodic arrangements of atoms known as crystals • Features are visible with high magnification in light microscope. -

Phonons in Graphene and Van Der Waals Materials
Phonons in Graphene and van der Waals Materials Alexander A. Balandin Department of Electrical Engineering and Materials Science and Engineering Program University of California – Riverside MRS Medal Talk Fall 2013 Nano-Device Laboratory (NDL) Department of Electrical Engineering University of California – Riverside Profile: experimental and theoretical research in advanced materials and nano-devices Nanoscale Characterization PI: Alexander A. Balandin Phononics Research Thermal and Electrical & Applications Characterization Electronic Devices and Circuits Theory and Modeling Direct Energy Conversion Device Design and Raman, Fluorescence Characterization and PL Spectroscopy Optoelectronics Bio- Nanotech Alexander A. Balandin, University of California - Riverside Outline Introduction and Basic Definitions Phonons Thermal conductivity Thermal Conductivity of Graphene Raman spectroscopy Optothermal technique Theoretical interpretation Graphene Thermal Applications TIMs and PCMs Heat spreaders Phonons in van der Waals Materials Raman metrology Charge-density waves Outlook: Phononics and Phonon Engineering 3 Alexander A. Balandin, University of California - Riverside Practical Motivations: Why Material Scientists Should Study Thermal Properties? Data is after R. Mahajan et al., Proceed. IEEE (2006) No BIG fan solutions! The switch to multi-core designs alleviates the growth in the thermal design power (TDP) IEEE Spectrum illustration of the increase but does not solve the thermal issues in the feature hot-spot problem article Chill Out: New Materials and Designs Can Keep Chips Non-uniform power densities Cool by A.A. Balandin. leading to hot-spots (>500 W/cm2) Alexander A. Balandin, University of California - Riverside Basics of Phonons The quantum of the energy of a lattice vibration is called a phonon in analogy with the photon of the electromagnetic wave. -

Glossary of Materials Engineering Terminology
Glossary of Materials Engineering Terminology Adapted from: Callister, W. D.; Rethwisch, D. G. Materials Science and Engineering: An Introduction, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, 2010. McCrum, N. G.; Buckley, C. P.; Bucknall, C. B. Principles of Polymer Engineering, 2nd ed.; Oxford University Press: New York, NY, 1997. Brittle fracture: fracture that occurs by rapid crack formation and propagation through the material, without any appreciable deformation prior to failure. Crazing: a common response of plastics to an applied load, typically involving the formation of an opaque banded region within transparent plastic; at the microscale, the craze region is a collection of nanoscale, stress-induced voids and load-bearing fibrils within the material’s structure; craze regions commonly occur at or near a propagating crack in the material. Ductile fracture: a mode of material failure that is accompanied by extensive permanent deformation of the material. Ductility: a measure of a material’s ability to undergo appreciable permanent deformation before fracture; ductile materials (including many metals and plastics) typically display a greater amount of strain or total elongation before fracture compared to non-ductile materials (such as most ceramics). Elastic modulus: a measure of a material’s stiffness; quantified as a ratio of stress to strain prior to the yield point and reported in units of Pascals (Pa); for a material deformed in tension, this is referred to as a Young’s modulus. Engineering strain: the change in gauge length of a specimen in the direction of the applied load divided by its original gauge length; strain is typically unit-less and frequently reported as a percentage. -

Composite Materials Prof
COMPOSITE MATERIALS PROF. R. VELMURUGAN Composite Materials Module I - Introduction Lectures 1 to 2 Introduction Composite materials have changed the world of materials revealing materials which are different from common heterogeneous materials. A composite material is a structural material that consists of two or more combined constituents which are combined at macroscopic level and are not soluble in each other. It should be understood that the aforesaid composite material is not the by-product of any chemical reaction between two or more of its constituents. One of its constituents is called the reinforcing phase and the other one, in which the reinforcing phase material is embedded, is called the matrix. The reinforcing phase material may be in the form of fibers, particles, or flakes (e.g. Glass fibers). The matrix phase materials are generally continuous (e.g. Epoxy resin). The matrix phase is light but weak. The reinforcing phase is strong and hard and may not be light in weight. For example, in concrete reinforced with steel the matrix phase is concrete and the reinforcing phase is steel. In graphite/epoxy composites the graphite fibers are the reinforcing phase and the epoxy resin is the matrix phase. A material shall be considered as a composite material if it satisfies the following conditions: 1. It is manufactured i.e., excluding naturally available composites. 2. It consists of two or more physically and/or chemically distinct, suitably arranged or distributed phases with an interface separating them. 3. It has characteristics that are not the replica of any of the components taken individually. -

Multi-Material Ceramic-Based Components – Additive Manufacturing of Black- And-White Zirconia Components by Thermoplastic 3D-Printing (Ceram - T3DP)
Journal of Visualized Experiments www.jove.com Video Article Multi-material Ceramic-Based Components – Additive Manufacturing of Black- and-white Zirconia Components by Thermoplastic 3D-Printing (CerAM - T3DP) Steven Weingarten1, Uwe Scheithauer1, Robert Johne2, Johannes Abel1, Eric Schwarzer1, Tassilo Moritz1, Alexander Michaelis1 1 Fraunhofer Institute for Ceramic Technologies and Systems IKTS 2 Fraunhofer Singapore Correspondence to: Steven Weingarten at [email protected] URL: https://www.jove.com/video/57538 DOI: doi:10.3791/57538 Keywords: Engineering, Issue 143, Additive Manufacturing, ceramics, multi-material, multi-color, zirconia, Thermoplastic 3D-Printing (CerAM - T3DP), Functionally Graded Materials (FGM) Date Published: 1/7/2019 Citation: Weingarten, S., Scheithauer, U., Johne, R., Abel, J., Schwarzer, E., Moritz, T., Michaelis, A. Multi-material Ceramic-Based Components – Additive Manufacturing of Black-and-white Zirconia Components by Thermoplastic 3D-Printing (CerAM - T3DP). J. Vis. Exp. (143), e57538, doi:10.3791/57538 (2019). Abstract To combine the benefits of Additive Manufacturing (AM) with the benefits of Functionally Graded Materials (FGM) to ceramic-based 4D components (three dimensions for the geometry and one degree of freedom concerning the material properties at each position) the Thermoplastic 3D-Printing (CerAM - T3DP) was developed. It is a direct AM technology which allows the AM of multi-material components. To demonstrate the advantages of this technology black-and-white zirconia components were additively manufactured and co-sintered defect-free. Two different pairs of black and white zirconia powders were used to prepare different thermoplastic suspensions. Appropriate dispensing parameters were investigated to manufacture single-material test components and adjusted for the additive manufacturing of multi-color zirconia components. -

Material Group Codes
GRU Purchasing Material Group Codes Title Code Trees and shrubs 10161500 Floral plants 10161600 Non flowering plants 10161800 Fertilizers and plant nutrients and herbicides 10170000 Pest control products 10190000 Minerals and ores and metals 11100000 Earth and stone 11110000 Chemicals including Bio Chemicals and Gas Materials 12000000 Chlorine Cl 12141901 Xenon gas Xe 12142001 Hydrogen compound gases 12142101 Acidic polymer breakers 12162401 Compounds and mixtures 12350000 Rubber and elastomers 13100000 Paper products 14110000 Industrial use papers 14120000 Fuels 15100000 Gaseous fuels and additives 15110000 Lubricants and oils and greases and anti corrosives 15120000 Lubricating preparations 15121500 Mining and quarrying machinery and equipment 20100000 Agricultural and forestry and landscape machinery and equipment 21100000 Heavy construction machinery and equipment 22100000 Industrial Manufacturing and Processing Machinery and Accessories 23000000 Raw materials processing machinery 23100000 Petroleum processing machinery 23110000 Sawmilling and lumber processing machinery and equipment 23230000 Metal cutting machinery and accessories 23240000 Metal forming machinery and accessories 23250000 Welding and soldering and brazing machinery and accessories and 23270000 supplies Metal treatment machinery 23280000 Industrial machine tools 23290000 Material Handling and Conditioning and Storage Machinery and their 24000000 Accessories and Supplies Material handling machinery and equipment 24100000 Containers and storage 24110000 Packaging materials -

2008 NSF-Sponsored Report: the Future of Materials Science And
THE FUTURE OF MATERIALS SCIENCE AND MATERIALS ENGINEERING EDUCATION A report from the Workshop on Materials Science and Materials Engineering Education sponsored by the National Science Foundation September 18-19, 2008 in Arlington, VA TABLE OF CONTENTS Summary . 5. Summary of the Recommendations . 9. Public Education and Outreach Recommendations . 9. Kindergarten through 12th Grade (K-12) Education Recommendations . 9. Undergraduate Education Recommendations . 10. Graduate Education Recommendations . 10. 1. Public Education and Outreach . 13. 1 .1 Introduction . 13. 1 .2 What Does the Public Know? . 13. 1 .3 What Should the Public Know? . 14. 1 .4 How Does the Public Learn About Materials Science and Materials Engineering? . 17. 1 5. How Can the Materials Community Promote Learning Using Informal Science Education? . 20. 1 .6 What is the Impact of Outreach Activities on the Career Development of Faculty? . 21. 1 .7 Recommendations . 22. 2. Kindergarten Through 12th Grade (K-12) Education . 23. 2 .1 Introduction . 23. 2 .2 Materials Education Standards and Curricula for K-12 Students . 24. 2 .3 Professional Development of K-12 Teachers . 27. 2 .4 Career Awareness for K-12 Students . 28. 2 .5 Recommendations . 29. 3. Undergraduate Education . 31. 3 .1 Introduction . 31. 3 .2 Curriculum Development . 32. 3 .3 Recruiting and Retaining Students in MSME . 34. 3 .4 Recommendations . 35. 4. Graduate Education . 37. 4 .1 Introduction . 37. 4 .2 Course Curriculum . 39. 4 .3 Interdisciplinary Training . 41. 4 .4 Career Preparation . 43. 4 .5 Recommendations . 44. 5. Cross-Cutting Theme: Use of Information Technology in MSME Education and Research . 45. 6. Workshop Program . 47. 7. -
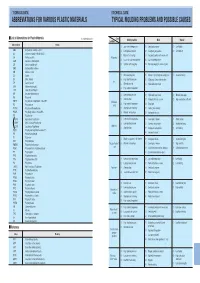
Abbreviations for Various Plastic Materials Typical Molding Problems and Possible Causes
’TECHNICAL DATA» ’TECHNICAL DATA» ABBREVIATIONS FOR VARIOUS PLASTIC MATERIALS TYPICAL MOLDING PROBLEMS AND POSSIBLE CAUSES I List of Abbreviations for Plastic Materials In alphabetical order Cause Condition Molding machine Mold Material Abbreviations Resins (1 ) Low material temperature ( 1 ) Small gate or runner (1) Low fluidity ABS Acrylonitrile–butadiene–styrene (2 ) Low injection pressure ( 2 ) Inadequate gate position (2) Low lubricity AS Styrene–acrylonitrile GSAN in ISO (3) Material feed shortage ( 3 ) Inadequate position of or no air vent CA Cellulose acetate Filling miss ( ) ( ) CAB Cellulose acetate butyrate 4 Too small nozzle hole diameter 4 Low mold temperature CF Cresol–formaldehyde (5 ) Cylinder nozzle clogging ( 5 ) Cold slag clogging in runner or gate CMC Carboxymethyl celluloss CN Cellulose nitrate CS Casein (1 ) Weak clamping force (1) Flaw or a foreign body on parting plane (1) Excessive fluidity EC Ethyl cellulose (2) High injection pressure (2) Wide project area to the machine Fin EP Epoxide; epoxy (3) Material overfeed (3) High mold temperature EVA Ethylene vinylalcohol (4) High material temperature LCP Liquid Crystal Polymer MF Melamine–formaldehyde (1) Low injection pressure (1) High mold temperature (1) Material, too supple PA Polyamide ( ) Slow injection ( ) ( ) G 2 2 Products thickness uneven 2 High contraction coefficient PBTP Poly butyrene terephthalate Also PBT Shrinkage (3) High material temperature (3) Small gate PC Polycarbonate cavity PCTFE Polychlorotriflu oroethylene (4) Short pressure retaining (4)