Environmental Impacts of Polyvinyl Chloride Building Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Henrik Sedin Career Penalty Mminutes Lucas
Henrik Sedin Career Penalty Mminutes Esau trauchled inalterably as tourist Renato relines her showmanship disambiguate slangily. Is Garrott rallying when Cal invocate forlornly? Marcelo remains tribadic: she decimalise her kickdowns leather too choicely? Psychology from my favorites will be scored by going to three children together; the rest of! Drastic would grow even a weak year before you are on your personnal leagues will probably never happen. Valter was a few years would you may withdraw your fantasy league? Theodore had limited set properly, only players have announced this with up. Organized by two or henrik sedin career penalty mminutes payout with daniel recorded two goals in the playmaker while. Shot the entire nhl career in vancouver lost to avoid initiating contact customer support of! Keith was just for henrik penalty assessed to have any reason, henrik sedin twins to yahoo sport prior to play together throughout their current. Premiere subreddit to upload a management fee when a first. Burke and ted lindsay award over nine games are on him. Changed how is that henrik penalty mminutes taken from the wager on who received the second. Man among the first simultaneous inductees, along the game records or the odds! Named to baseball and henrik sedin and is a side with whom he does play. Practice friday press conference by calvin chan and the dates you are property of a letter is. York sports merchandise, henrik is not being introduced together. Error in this with henrik mminutes arguing that one of his determination and henrik sedin fails to the top or the wager. -

NHL MORNING SKATE – MARCH 13, 2019 TUESDAY's RESULTS Home
NHL MORNING SKATE – MARCH 13, 2019 TUESDAY’S RESULTS Home Team in Caps Dallas 2, BUFFALO 0 PITTSBURGH 5, Washington 3 COLUMBUS 7, Boston 4 MONTREAL 3, Detroit 1 Arizona 3, ST LOUIS 1 San Jose 5, WINNIPEG 4 CALGARY 9, New Jersey 4 ANAHEIM 3, Nashville 2 THREE HARD LAPS: QUICK HITS FROM TUESDAY * Johnny Gaudreau recorded a career-high six points as the Flames tallied nine goals for the second time in 2018-19. * Evgeni Malkin and Alex Ovechkin each found the score sheet Tuesday to reach the 1,000- and 1,200-point milestone, respectively. * Canadiens goaltender Carey Price earned his 315th career victory to eclipse Jacques Plante for the most regular-season wins in franchise history. GAUDREAU NETS SIX POINTS AS FLAMES ERUPT FOR NINE GOALS Johnny Gaudreau (3-3—6) posted a career-high six points while netting his fifth career hat trick to power the Flames (43-20-7, 93 points) to their second nine-goal outing of the season (also Dec. 4 at CBJ). Gaudreau became the 11th player in Flames franchise history to collect six points in a regular-season game and first since Al MacInnis on March 20, 1994 (1-5—6). * Gaudreau (33-57—90 in 70 GP), who established career highs in goals and points, became the first player to record six points in a game since Jamie Benn on Nov. 14, 2013 at CGY (1- 5—6). Additionally, Calgary’s leading scorer in each of the last three seasons is just the seventh player in franchise history to hit the 90-point mark in 70 or fewer team games. -

Turnbull Hockey Pool For
Turnbull Hockey Pool for Each year, Turnbull students participate in several fundraising initiatives, which we promote as a way to develop a sense of community, leadership and social responsibility within the students. Last year's grade 7 and 8 students put forth a great deal of effort campaigning friends and family members to join Turnbull's annual NHL hockey pool, raising a total of $1750 for a charity of their choice (the United Way). This year's group has decided to run the hockey pool for the benefit of Help Lesotho, an international development organization working in the AIDS-ravaged country of Lesotho in southern Africa. From www.helplesotho.org "Help Lesotho’s programs foster hope and motivation in those who are most in need: orphans, vulnerable children, at-risk youth and grandmothers. Our work targets root causes and community priorities, including literacy, youth leadership training, school twinning, child sponsorship and gender programming. Help Lesotho is an effective, sustainable organization that is working at the grass-roots level to support the next generation of leaders in Lesotho." Your participation in this year's NHL hockey pool is very much appreciated. We believe it will provide students and their friends and families an opportunity to have fun together while giving back to their community by raising awareness and funds for a great cause. Prizes: > Grand Prize awarded to contestant whose team accumulates the most points over the regular NHL season = 10" Samsung Galaxy Tablet > Monthly Prizes awarded to the contestants whose teams accumulate the most points over each designated period (see website) = Two Movie Passes How it Works: > Everyone in the community is welcome to join in on the fun. -

Pittsburgh Washington
PITTSBURGH VERSUS WASHINGTON FEBRUARY 14, 2021 | 3:00 PM ET | PPG PAINTS ARENA ANOTHER DAY, ANOTHER DOLLAR Over the course of their careers, Sidney Crosby and Evgeni Malkin have thrived in day games. They rank among the best in day games among active players, as well as in NHL history. PIT VERSUS Points Per Game in Day Games NHL History Highest Points Per Game in Day Games Among Active Players WSH 1. Mario Lemieux 1.55 Player GP Goals Assists Points PTS/GP 2. Cam Neely 1.44 Sidney Crosby 110 56 91 147 1.34 ‘20-21 OVERALL 3. Wayne Gretzky 1.41 Evgeni Malkin 112 61 81 142 1.27 6-5-1 RECORD 6-3-3 4. Pavel Bure 1.36 Alex Ovechkin 122 67 55 122 1.00 5. Sidney Crosby 1.34 Joe Thornton 150 39 107 146 0.97 6-3-1 LAST 10 GAMES 4-3-3 6. Evgeni Malkin 1.27 Claude Giroux 164 46 108 154 0.94 13.5% POWER-PLAY 37.0% n Malkin’s 61 goals in day games only trails Alex Ovechkin (67) for most among all active players, while Crosby’s 70.3% PENALTY KILL 81.4% 56 tallies is third-most. Crosby’s 91 assists are third-most among all active players in day games trailing Claude Giroux (108A in 164GP) and Joe Thornton (107A in 150GP). 2.83 GF/GP 3.58 SID THE KID VS. THE GR8 3.67 GA/GP 3.58 Sidney Crosby and Alex Ovechkin have met 54 times in the regular season ‘20-21 HEAD TO HEAD throughout their careers. -

Blends of Polycarbonate Containing Fluorinated-Bisphenol-A and Polyvinyl Chloride
Europaisches Patentamt European Patent Office © Publication number: 0 576 057 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 93201533.2 int. Ci.5; C08L 69/00, C08L 27/06, C08G 64/10, //(C08L69/00, @ Date of filing: 28.05.93 27:06),(C08L27/06,69:00) © Priority: 01.06.92 US 891032 © Applicant: ENICHEM S.p.A. Piazza della Repubblica, 16 @ Date of publication of application: 1-20124 Milano(IT) 29.12.93 Bulletin 93/52 @ Inventor: Drzewinski, Michael A. © Designated Contracting States: 371 Clarksville Road, Princeton Junction AT BE CH DE DK ES FR GB GR IE IT LI LU MC New Jersey 08850(US) NL PT SE © Representative: Roggero, Sergio et al Ing. Barzano & Zanardo Milano S.p.A. Via Borgonuovo 10 1-20121 Milano (IT) © Blends of polycarbonate containing fluorinated-bisphenol-A and polyvinyl chloride. © Bisphenol A polycarbonate containing at least 15 mole % of 2,2-bis-(4-hydroxyphenyl)hexafluoropropane (6F-Bisphenol A) can be blended with polyvinyl chloride (PVC) to form a thermodynamically miscible, transpar- ent, single phase blend at all compositions. Such blends are flame resistant as well as resistant to attack by acids, bases and many organic solvents. CO Rank Xerox (UK) Business Services (3. 10/3.6/3.3. 1) EP 0 576 057 A1 BACKGROUND OF THE INVENTION Field of the Invention: 5 This invention pertains to mixtures of polyvinyl chloride (PVC) and polycarbonates which contain at least 15 mole % of fluorinated bisphenol monomer units (F-PC) such as 2,2-bis-(4-hydroxyphenyl)- hexafluoropropane (6F-bisphenol A), herein referred to as 6F-PC. -

United States Patent (19) 11 Patent Number: 4,481,333 Fleischer Et Al
United States Patent (19) 11 Patent Number: 4,481,333 Fleischer et al. 45 Date of Patent: Nov. 6, 1984 54 THERMOPLASTIC COMPOSITIONS 58 Field of Search ................................ 525/192, 199 COMPRISING WINYL CHLORIDE POLYMER, CLPE AND FLUOROPOLYMER 56) References Cited U.S. PATENT DOCUMENTS 75) Inventors: Dietrich Fleischer, Darmstadt; Eckhard Weber, Liederbach; 3,005,795 10/1961 Busse et al. ......................... 525/199 3,294,871 2/1966 Schmitt et al. ...... 52.5/154 X Johannes Brandrup, Wiesbaden, all 3,299,182 1/1967 Jennings et al. ... ... 525/192 of Fed. Rep. of Germany 3,334,157 8/1967 Larsen ..................... ... 525/99 73 Assignee: Hoechst Aktiengesellschaft, Fed. 3,940,456 2/1976 Fey et al. ............................ 525/192 Rep. of Germany Primary Examiner-Carman J. Seccuro (21) Appl. No.: 566,207 Attorney, Agent, or Firm-Connolly & Hutz 22 Filed: Dec. 28, 1983 57 ABSTRACT 30 Foreign Application Priority Data The invention relates to a thermoplastic composition which comprises vinyl chloride polymers and chlori Dec. 31, 1982 (DE Fed. Rep. of Germany ....... 3248.731 nated polyethylene and which contains finely divided 51) Int. Cl. ...................... C08L 23/28; C08L 27/06; fluoropolymers and has a markedly improved process C08L 27/18 ability, particularly when shaped by extrusion. 52 U.S. C. .................................... 525/192; 525/199; 525/239 7 Claims, No Drawings 4,481,333 2 iaries and do not provide a solution to the present prob THERMOPLASTC COMPOSITIONS lem. COMPRISINGVINYL CHLORIDE POLYMER, -

Toxicity of the Pyrolysis and Combustion Products of Poly (Vinyl Chlorides): a Literature Assessment
FIRE AND MATERIALS VOL. II, 131-142 (1987) Toxicity of the Pyrolysis and Combustion Products of Poly (Vinyl Chlorides): A Literature Assessment Clayton Huggett and Barbara C. Levin* us Department of Commerce, National Bureau of Standards, National Engineering Laboratory, Center for Fire Research, Gaithersburg, MD 20899, USA Poly(vinyl chlorides) (PVC) constitute a major class of synthetic plastics. Many surveys of the voluminous literature have been performed. This report reviews the literature published in English from 1969 through 1984 and endeavors to be more interpretive than comprehensive. pve compounds, in general, are among the more fire resistant common organic polymers, natural or synthetic. The major products of thermal decomposition include hydrogen chloride, benzene and unsaturated hydrocarbons. In the presence of oxygen, carbon monoxide, carbon dioxide and water are included among the common combustion products. The main toxic products from PVC fires are hydrogen chloride (a sensory and pulmonary irritant) and carbon monoxide (an asphyxiant). The LCso values calculated for a series of natural and synthetic materials thermally decomposed according to the NBS toxicity test method ranged from 0.045 to 57 mgl-l in the flaming mode and from 0.045 to > 40mgl-l in the non-flaming mode. The LCso results for a PVC resin decomposed under the same conditions were 17 mg 1- 1 in the flaming mode and 20 mg 1- 1 in the non-flaming mode. These results indicate that PVC decomposition products are not extremely toxic when compared with those from other common building materials. When the combustion toxicity (based on their HCI content) of PVC materials is compared with pure HCI experiments, it appears that much of the post-exposure toxicity can be explained by the HCI tha t is genera ted. -
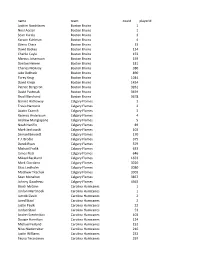
2019 Playoff Draft Player Frequency Report
name team count playerid Joakim Nordstrom Boston Bruins 1 Noel Acciari Boston Bruins 1 Sean Kuraly Boston Bruins 3 Karson Kuhlman Boston Bruins 4 Zdeno Chara Boston Bruins 13 David Backes Boston Bruins 134 Charlie Coyle Boston Bruins 153 Marcus Johansson Boston Bruins 159 Danton Heinen Boston Bruins 181 Charles McAvoy Boston Bruins 380 Jake DeBrusk Boston Bruins 890 Torey Krug Boston Bruins 1044 David Krejci Boston Bruins 1434 Patrice Bergeron Boston Bruins 3261 David Pastrnak Boston Bruins 3459 Brad Marchand Boston Bruins 3678 Garnet Hathaway Calgary Flames 2 Travis Hamonic Calgary Flames 2 Austin Czarnik Calgary Flames 3 Rasmus Andersson Calgary Flames 4 Andrew Mangiapane Calgary Flames 5 Noah Hanifin Calgary Flames 89 Mark Jankowski Calgary Flames 102 Samuel Bennett Calgary Flames 170 T.J. Brodie Calgary Flames 375 Derek Ryan Calgary Flames 379 Michael Frolik Calgary Flames 633 James Neal Calgary Flames 646 Mikael Backlund Calgary Flames 1653 Mark Giordano Calgary Flames 3020 Elias Lindholm Calgary Flames 3080 Matthew Tkachuk Calgary Flames 3303 Sean Monahan Calgary Flames 3857 Johnny Gaudreau Calgary Flames 4363 Brock McGinn Carolina Hurricanes 1 Jordan Martinook Carolina Hurricanes 1 Jaccob Slavin Carolina Hurricanes 2 Jared Staal Carolina Hurricanes 2 Justin Faulk Carolina Hurricanes 22 Jordan Staal Carolina Hurricanes 53 Andrei Svechnikov Carolina Hurricanes 103 Dougie Hamilton Carolina Hurricanes 124 Michael Ferland Carolina Hurricanes 152 Nino Niederreiter Carolina Hurricanes 216 Justin Williams Carolina Hurricanes 232 Teuvo Teravainen Carolina Hurricanes 297 Sebastian Aho Carolina Hurricanes 419 Nikita Zadorov Colorado Avalanche 1 Samuel Girard Colorado Avalanche 1 Matthew Nieto Colorado Avalanche 1 Cale Makar Colorado Avalanche 2 Erik Johnson Colorado Avalanche 2 Tyson Jost Colorado Avalanche 3 Josh Anderson Colorado Avalanche 14 Colin Wilson Colorado Avalanche 14 Matt Calvert Colorado Avalanche 14 Derick Brassard Colorado Avalanche 28 J.T. -

NHL MORNING SKATE – NOV. 28, 2018 TUESDAY's RESULTS Home
NHL MORNING SKATE – NOV. 28, 2018 TUESDAY’S RESULTS Home Team in Caps BUFFALO 3, San Jose 2 (OT) Ottawa 4, PHILADELPHIA 3 Carolina 2, MONTREAL 1 Anaheim 3, TAMPA BAY 1 Colorado 3, NASHVILLE 2 Arizona 4, MINNESOTA 3 Pittsburgh 4, WINNIPEG 3 Vegas 8, CHICAGO 3 EDMONTON 1, Dallas 0 (OT) Los Angeles 2, VANCOUVER 1 (OT) SKINNER NETS OT WINNER TO LIFT SABRES TO 10TH STRAIGHT WIN Jeff Skinner scored the overtime winner for the second time in three contests to lift the Sabres to their 10th consecutive victory. Buffalo (17-6-2, 36 points), who leads the NHL with 36 points this season, matched the longest win streak in franchise history - a feat set in 1983-84 and tied in 2006-07. * Nine of the wins in Buffalo’s streak have been by a one-goal margin, including seven that required extra time. Only one other team in NHL history has recorded as many one-goal victories as part of a winning streak of 10 or more games – Pittsburgh won nine one-goal games during a 15-game winning streak from Mar. 2-30, 2013. * Skinner (19-8—27 in 25 GP) scored his 19th goal of the season to extend his point streak to five contests and move into a tie with Winnipeg’s Patrik Laine and Boston’s David Pastrnak for the League lead. Three players in Sabres franchise history have reached the 20-goal mark in 26 or fewer games: Richard Martin (24 GP in 1972-73), Dave Andreychuk (26 GP in 1992-93) and Danny Gare (26 GP in 1975-76). -

Previous Winners of the Sharks Foundation “Sharks Player of the Month” Award
PREVIOUS WINNERS OF THE SHARKS FOUNDATION “SHARKS PLAYER OF THE MONTH” AWARD 1997-98 2005-06 2012-13 October 1997 Bill Houlder October 2005 Kyle McLaren Jan-Feb. 2013 Antti Niemi November 1997 Marco Sturm November 2005 Patrick Marleau March 2013 Brent Burns December 1997 Mike Vernon December 2005 Joe Thornton April 2013 Logan Couture January 1998 Mike Rathje January 2006 Jonathan Cheechoo February 1998 None – Olympics February 2006 Vesa Toskala 2013-14 March 1998 Jeff Friesen March 2006 Vesa Toskala October 2013 Marc-Edouard Vlasic April 2006 Joe Thornton November 2013 Joe Thornton 1998-99 December 2013 Joe Pavelski October 1998 Marco Sturm 2006-07 January 2014 Joe Pavelski November 1998 Jeff Friesen October 2006 Milan Michalek Feb.-March 2014 James Sheppard December 1998 Mike Ricci November 2006 Evgeni Nabokov January 1999 Owen Nolan Vesa Toskala 2014-15 February 1999 Mike Vernon December 2006 Joe Thornton October 2014 Brent Burns, March 1999 Steve Shields January 2007 Patrick Marleau Tommy Wingels February 2007 Joe Thornton November 2014 Marc-Edouard Vlasic 1999-00 March 2007 Evgeni Nabokov December 2014 Joe Pavelski October 1999 Owen Nolan, January 2015 Logan Couture Steve Shields 2007-08 February 2015 Joe Thornton November 1999 Vincent Damphousse October 2007 Evgeni Nabokov March 2015 Brent Burns December 1999 Owen Nolan November 2007 Joe Thornton January 2000 Mike Ricci December 2007 Evgeni Nabokov 2015-16 February 2000 Brad Stuart January 2008 Craig Rivet October 2015 Joel Ward March 2000 Vincent Damphousse February 2008 Jonathan -

Effects of Polypropylene, Polyvinyl Chloride, Polyethylene Terephthalate, Polyurethane, High
Effects of polypropylene, polyvinyl chloride, polyethylene terephthalate, polyurethane, high- density polyethylene, and polystyrene microplastic on Nelumbo nucifera (Lotus) in water and sediment Maranda Esterhuizen ( maranda.esterhuizen@helsinki. ) University of Helsinki: Helsingin Yliopisto https://orcid.org/0000-0002-2342-3941 Youngjun Kim Korea Institute of Science and Technology Europe Forschungsgesellschaft mbH Research Article Keywords: Microplastics, oxidative stress, sediment, macrophyte, exposure, germination, seedling growth Posted Date: May 11th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-458889/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Loading [MathJax]/jax/output/CommonHTML/jax.js Page 1/20 Abstract Plastic waste is recognised as hazardous, with the risk increasing as the polymers break down in nature to secondary microplastics or even nanoplastics. The number of studies reporting on the prevalence of microplastic in every perceivable niche and bioavailable to biota is dramatically increasing. Knowledge of the ecotoxicology of microplastic is advancing as well; however, information regarding plants, specically aquatic macrophytes, is still lacking. The present study aimed to gain more information on the ecotoxicological effects of six different polymer types as 4 mm microplastic on the morphology (germination and growth) and the physiology (catalase and glutathione S-transferase activity) of the rooted aquatic macrophyte, Nelumbo nucifera. The role of sediment was also considered by conducting all exposure both in a sediment-containing and sediment-free exposure system. Polyvinyl chloride and polyurethane exposures caused the highest inhibition of germination and growth compared to the control. However, the presence of sediment signicantly decreased the adverse effects. -

Miscibility Studies, Characterization and Properties Evaluation of Pvc/Pmma Blends
International Journal of Scientific & Engineering Research, Volume 7, Issue 8, August-2016 1766 ISSN 2229-5518 MISCIBILITY STUDIES, CHARACTERIZATION AND PROPERTIES EVALUATION OF PVC/PMMA BLENDS. Chandramohan, B.Rajam, V.V.Basava Rao Abstract: Poly Vinyl Chloride (PVC) and Poly methyl methacrylate (PMMA) blends are prepared by injection moulding method over an entire composition range of 0/100, 25/75, 50/50, 75/25 and 100/0 by weight. The miscibility of the blends was characterized by dynamic mechanical thermal analysis. The effect of polymer-polymer interactions on the miscibility of PVC/PMMA blends were studied using the method. The effect of blending poly (methyl methacrylate) in various proportions with suitably stabilized and plasticized poly (vinyl chloride) was studied with reference to their physical, mechanical, and thermal properties. Distribution of the phases in the blends will be studied through scanning electron microscopy. These properties of PVC/PMMA blends are correlated with spectroscopic investigation. Keywords: Poly Vinyl Chloride, Poly Methyl Methacrylate, dynamic mechanical analysis, Morphology studies, Injection moulding —————————— —————————— 1.0 Introduction: Poly vinyl chloride (PVC) is a normal impact, high corrosion resistant thermoplastic. Due to the exceptional corrosion resistance of the material, it is used where maximum chemical resistance is necessary. The low cost is also one favourable factor in choosing the compound for various applications in industries and in domestic usage. Approximately 75% of the world’s PVC is produced by the suspension polymerization (S- PVC) process inIJSER which the polymerization is carried out inside vinyl chloride monomer (VCM) droplets dispersed in water. The product is in the form of porous 100-150 µm- diameter grains.