Effects of Polypropylene, Polyvinyl Chloride, Polyethylene Terephthalate, Polyurethane, High
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Properties of Polypropylene Yarns with a Polytetrafluoroethylene
coatings Article Properties of Polypropylene Yarns with a Polytetrafluoroethylene Coating Containing Stabilized Magnetite Particles Natalia Prorokova 1,2,* and Svetlana Vavilova 1 1 G.A. Krestov Institute of Solution Chemistry of the Russian Academy of Sciences, Akademicheskaya St. 1, 153045 Ivanovo, Russia; [email protected] 2 Department of Natural Sciences and Technosphere Safety, Ivanovo State Polytechnic University, Sheremetevsky Ave. 21, 153000 Ivanovo, Russia * Correspondence: [email protected] Abstract: This paper describes an original method for forming a stable coating on a polypropylene yarn. The use of this method provides this yarn with barrier antimicrobial properties, reducing its electrical resistance, increasing its strength, and achieving extremely high chemical resistance, similar to that of fluoropolymer yarns. The method is applied at the melt-spinning stage of polypropylene yarns. It is based on forming an ultrathin, continuous, and uniform coating on the surface of each of the yarn filaments. The coating is formed from polytetrafluoroethylene doped with magnetite nanoparticles stabilized with sodium stearate. The paper presents the results of a study of the effects of such an ultrathin polytetrafluoroethylene coating containing stabilized magnetite particles on the mechanical and electrophysical characteristics of the polypropylene yarn and its barrier antimicrobial properties. It also evaluates the chemical resistance of the polypropylene yarn with a coating based on polytetrafluoroethylene doped with magnetite nanoparticles. Citation: Prorokova, N.; Vavilova, S. Properties of Polypropylene Yarns Keywords: coatings; polypropylene yarn; polytetrafluoroethylene; magnetite nanoparticles; barrier with a Polytetrafluoroethylene antimicrobial properties; surface electrical resistance; chemical resistance; tensile strength Coating Containing Stabilized Magnetite Particles. Coatings 2021, 11, 830. https://doi.org/10.3390/ coatings11070830 1. -

POLYPROPYLENE Chemical Resistance Guide
POLYPROPYLENE Chemical Resistance Guide SECOND EDITION PP CHEMICAL RESISTANCE GUIDE Thermoplastics: Polypropylene (PP) Chemical Resistance Guide Polypropylene (PP) 2nd Edition © 2020 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 About IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. xx: Max Recommended Temperature – Unsuitable / Insufficient Data A: Applicable in Some Cases, consult IPEX 2 IPEX Chemical Resistance Guide for PP INTRODUCTION Thermoplastics and elastomers have outstanding resistance to a wide range of chemical reagents. The chemical resistance of plastic piping is basically a function of the thermoplastic material and the compounding components. -

Blends of Polycarbonate Containing Fluorinated-Bisphenol-A and Polyvinyl Chloride
Europaisches Patentamt European Patent Office © Publication number: 0 576 057 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 93201533.2 int. Ci.5; C08L 69/00, C08L 27/06, C08G 64/10, //(C08L69/00, @ Date of filing: 28.05.93 27:06),(C08L27/06,69:00) © Priority: 01.06.92 US 891032 © Applicant: ENICHEM S.p.A. Piazza della Repubblica, 16 @ Date of publication of application: 1-20124 Milano(IT) 29.12.93 Bulletin 93/52 @ Inventor: Drzewinski, Michael A. © Designated Contracting States: 371 Clarksville Road, Princeton Junction AT BE CH DE DK ES FR GB GR IE IT LI LU MC New Jersey 08850(US) NL PT SE © Representative: Roggero, Sergio et al Ing. Barzano & Zanardo Milano S.p.A. Via Borgonuovo 10 1-20121 Milano (IT) © Blends of polycarbonate containing fluorinated-bisphenol-A and polyvinyl chloride. © Bisphenol A polycarbonate containing at least 15 mole % of 2,2-bis-(4-hydroxyphenyl)hexafluoropropane (6F-Bisphenol A) can be blended with polyvinyl chloride (PVC) to form a thermodynamically miscible, transpar- ent, single phase blend at all compositions. Such blends are flame resistant as well as resistant to attack by acids, bases and many organic solvents. CO Rank Xerox (UK) Business Services (3. 10/3.6/3.3. 1) EP 0 576 057 A1 BACKGROUND OF THE INVENTION Field of the Invention: 5 This invention pertains to mixtures of polyvinyl chloride (PVC) and polycarbonates which contain at least 15 mole % of fluorinated bisphenol monomer units (F-PC) such as 2,2-bis-(4-hydroxyphenyl)- hexafluoropropane (6F-bisphenol A), herein referred to as 6F-PC. -

United States Patent (19) 11 Patent Number: 4,481,333 Fleischer Et Al
United States Patent (19) 11 Patent Number: 4,481,333 Fleischer et al. 45 Date of Patent: Nov. 6, 1984 54 THERMOPLASTIC COMPOSITIONS 58 Field of Search ................................ 525/192, 199 COMPRISING WINYL CHLORIDE POLYMER, CLPE AND FLUOROPOLYMER 56) References Cited U.S. PATENT DOCUMENTS 75) Inventors: Dietrich Fleischer, Darmstadt; Eckhard Weber, Liederbach; 3,005,795 10/1961 Busse et al. ......................... 525/199 3,294,871 2/1966 Schmitt et al. ...... 52.5/154 X Johannes Brandrup, Wiesbaden, all 3,299,182 1/1967 Jennings et al. ... ... 525/192 of Fed. Rep. of Germany 3,334,157 8/1967 Larsen ..................... ... 525/99 73 Assignee: Hoechst Aktiengesellschaft, Fed. 3,940,456 2/1976 Fey et al. ............................ 525/192 Rep. of Germany Primary Examiner-Carman J. Seccuro (21) Appl. No.: 566,207 Attorney, Agent, or Firm-Connolly & Hutz 22 Filed: Dec. 28, 1983 57 ABSTRACT 30 Foreign Application Priority Data The invention relates to a thermoplastic composition which comprises vinyl chloride polymers and chlori Dec. 31, 1982 (DE Fed. Rep. of Germany ....... 3248.731 nated polyethylene and which contains finely divided 51) Int. Cl. ...................... C08L 23/28; C08L 27/06; fluoropolymers and has a markedly improved process C08L 27/18 ability, particularly when shaped by extrusion. 52 U.S. C. .................................... 525/192; 525/199; 525/239 7 Claims, No Drawings 4,481,333 2 iaries and do not provide a solution to the present prob THERMOPLASTC COMPOSITIONS lem. COMPRISINGVINYL CHLORIDE POLYMER, -

Toxicity of the Pyrolysis and Combustion Products of Poly (Vinyl Chlorides): a Literature Assessment
FIRE AND MATERIALS VOL. II, 131-142 (1987) Toxicity of the Pyrolysis and Combustion Products of Poly (Vinyl Chlorides): A Literature Assessment Clayton Huggett and Barbara C. Levin* us Department of Commerce, National Bureau of Standards, National Engineering Laboratory, Center for Fire Research, Gaithersburg, MD 20899, USA Poly(vinyl chlorides) (PVC) constitute a major class of synthetic plastics. Many surveys of the voluminous literature have been performed. This report reviews the literature published in English from 1969 through 1984 and endeavors to be more interpretive than comprehensive. pve compounds, in general, are among the more fire resistant common organic polymers, natural or synthetic. The major products of thermal decomposition include hydrogen chloride, benzene and unsaturated hydrocarbons. In the presence of oxygen, carbon monoxide, carbon dioxide and water are included among the common combustion products. The main toxic products from PVC fires are hydrogen chloride (a sensory and pulmonary irritant) and carbon monoxide (an asphyxiant). The LCso values calculated for a series of natural and synthetic materials thermally decomposed according to the NBS toxicity test method ranged from 0.045 to 57 mgl-l in the flaming mode and from 0.045 to > 40mgl-l in the non-flaming mode. The LCso results for a PVC resin decomposed under the same conditions were 17 mg 1- 1 in the flaming mode and 20 mg 1- 1 in the non-flaming mode. These results indicate that PVC decomposition products are not extremely toxic when compared with those from other common building materials. When the combustion toxicity (based on their HCI content) of PVC materials is compared with pure HCI experiments, it appears that much of the post-exposure toxicity can be explained by the HCI tha t is genera ted. -

Development, Structure and Strength Properties of PP/PMMA/FA Blends
Bull. Mater. Sci., Vol. 23, No. 2, April 2000, pp. 103–107. © Indian Academy of Sciences. Development, structure and strength properties of PP/PMMA/FA blends NAVIN CHAND* and S R VASHISHTHA Regional Research Laboratory, Habibganj Naka, Bhopal 462 026, India MS received 10 September 1999; revised 29 January 2000 Abstract. A new type of flyash filled PP/PMMA blend has been developed. Structural and thermal properties of flyash (FA) filled polypropylene (PP)/polymethyl methacrylate (PMMA) blend system have been determined and analysed. Filled polymer blends were developed on a single screw extruder. Strength and thermal pro- perties of FA filled and unfilled PP/PMMA blends were determined. Addition of flyash imparted dimensional and thermal stability, which has been observed in scanning electron micrographs and in TGA plot. Increase of flyash concentration increased the initial degradation temperature of PP/PMMA blend. The increase of thermal stability has been explained based on increased mechanical interlocking of PP/PMMA chains inside the hollow structure of flyash. Keywords. Flyash polymer composite; morphology; particulate. 1. Introduction polarity and the polymer with polar groups facilitate sur- face bonding because the surface of filler can be easily Polypropylene (PP) is a commercially important polymer, wetted by a polymer. Therefore, whether or not the poly- which is of practical use in a wide range of applications mer molecule has polarity is an important parameter in (Glenz 1986). Its morphology (Frank 1968) means that determining the reinforcement effect of polymer compo- the mechanical properties of PP are moderate, so if one sites. Chemical bonding between polymer matrix and wants to extend the field of application of this material, an filler offers good bonding of constituents. -

Wood Fiber Reinforcement of Styrene-Maleic Anhydride Copolymers
The Fourth International Conference on Woodfiber-Plastic Composites Wood Fiber Reinforcement of Styrene-Maleic Anhydride Copolymers John Simonsen Rodney Jacobson Roger Rowell Abstract Styrene-maleic anhydride (SMA) copolymers are We also investigated the thermal properties of the used in the automotive industry, primarily for interior composites using differential scanning calorimetry. parts. The use of wood fillers may provide a more While the SMA molecule contains an anhydride economical means of manufacturing these items group, we observed no evidence of chemical bonding and/or improve their properties. We made a prelimi- between SMA and the wood filler. nary study of the feasibility of utilizing wood-based Introduction fillers in SMA copolymers, comparing the mechanical The development of useful wood-filled thermo- properties of wood-filled SMA to wood-filled poly- styrene. The fillers studied were fibers for aspen plastic products has received increasing attention medium density fiberboard, pine wood flour, and from manufacturers and the research community milled recycled newsprint, which showed unusually recently. This is due to the low cost and easy proc- high properties considering its size and aspect ratio. essability of wood fillers. In addition, wood fillers can add reinforcement in some cases, improving the properties of the final composite over those of the unfilled plastic (6, 7, 10–12). Simonsen: Styrene-maleic anhydride (SMA) copolymers are Assistant Professor, Dept- of Forest Prod., Oregon State utilized in the automotive industry for the injection University, Corvallis, Oregon molding and thermoforming of interior parts, The Jacobson: superiority of SMA over polystyrene is due to its Materials Engineer, USDA Forest Serv., Forest Prod. -

Miscibility Studies, Characterization and Properties Evaluation of Pvc/Pmma Blends
International Journal of Scientific & Engineering Research, Volume 7, Issue 8, August-2016 1766 ISSN 2229-5518 MISCIBILITY STUDIES, CHARACTERIZATION AND PROPERTIES EVALUATION OF PVC/PMMA BLENDS. Chandramohan, B.Rajam, V.V.Basava Rao Abstract: Poly Vinyl Chloride (PVC) and Poly methyl methacrylate (PMMA) blends are prepared by injection moulding method over an entire composition range of 0/100, 25/75, 50/50, 75/25 and 100/0 by weight. The miscibility of the blends was characterized by dynamic mechanical thermal analysis. The effect of polymer-polymer interactions on the miscibility of PVC/PMMA blends were studied using the method. The effect of blending poly (methyl methacrylate) in various proportions with suitably stabilized and plasticized poly (vinyl chloride) was studied with reference to their physical, mechanical, and thermal properties. Distribution of the phases in the blends will be studied through scanning electron microscopy. These properties of PVC/PMMA blends are correlated with spectroscopic investigation. Keywords: Poly Vinyl Chloride, Poly Methyl Methacrylate, dynamic mechanical analysis, Morphology studies, Injection moulding —————————— —————————— 1.0 Introduction: Poly vinyl chloride (PVC) is a normal impact, high corrosion resistant thermoplastic. Due to the exceptional corrosion resistance of the material, it is used where maximum chemical resistance is necessary. The low cost is also one favourable factor in choosing the compound for various applications in industries and in domestic usage. Approximately 75% of the world’s PVC is produced by the suspension polymerization (S- PVC) process inIJSER which the polymerization is carried out inside vinyl chloride monomer (VCM) droplets dispersed in water. The product is in the form of porous 100-150 µm- diameter grains. -

Functionalization of Polyethylene (PE) and Polypropylene (PP) Material Using Chitosan Nanoparticles with Incorporated Resveratrol As Potential Active Packaging
materials Article Functionalization of Polyethylene (PE) and Polypropylene (PP) Material Using Chitosan Nanoparticles with Incorporated Resveratrol as Potential Active Packaging Tjaša Kraševac Glaser 1,*, Olivija Plohl 1, Alenka Vesel 2, Urban Ajdnik 1 , Nataša Poklar Ulrih 3, Maša Knez Hrnˇciˇc 4, Urban Bren 4 and Lidija Fras Zemljiˇc 1,* 1 Laboratory for Characterization and Processing of Polymers, Faculty of Mechanical Engineering, University of Maribor, Smetanova 17, SI-2000 Maribor, Slovenia 2 Department of Surface Engineering and Optoelectronics, Jožef Stefan Institute, Teslova 30, SI-1000 Ljubljana, Slovenia 3 Department of Food Science and Technology, Biotechnical Faculty, University of Ljubljana, Jamnikarjeva 101, SI-1000 Ljubljana, Slovenia 4 Faculty of Chemistry and Chemical Engineering, University of Maribor, Smetanova 17, SI-2000 Maribor, Slovenia * Correspondence: [email protected] (T.K.G.); [email protected] (L.F.Z.); Tel.: +386-2-220-7607 (T.G.K.); +386-2-220-7909 (L.F.Z.) Received: 21 May 2019; Accepted: 28 June 2019; Published: 1 July 2019 Abstract: The present paper reports a novel method to improve the properties of polyethylene (PE) and polypropylene (PP) polymer foils suitable for applications in food packaging. It relates to the adsorption of chitosan-colloidal systems onto untreated and oxygen plasma-treated foil surfaces. It is hypothesized that the first coated layer of chitosan macromolecular solution enables excellent antibacterial properties, while the second (uppermost) layer contains a network of polyphenol resveratrol, embedded into chitosan nanoparticles, which enables antioxidant and antimicrobial properties simultaneously. X-ray photon spectroscopy (XPS) and infrared spectroscopy (FTIR) showed successful binding of both coatings onto foils as confirmed by gravimetric method. -

Bio-Based and Biodegradable Plastics – Facts and Figures Focus on Food Packaging in the Netherlands
Bio-based and biodegradable plastics – Facts and Figures Focus on food packaging in the Netherlands Martien van den Oever, Karin Molenveld, Maarten van der Zee, Harriëtte Bos Rapport nr. 1722 Bio-based and biodegradable plastics - Facts and Figures Focus on food packaging in the Netherlands Martien van den Oever, Karin Molenveld, Maarten van der Zee, Harriëtte Bos Report 1722 Colophon Title Bio-based and biodegradable plastics - Facts and Figures Author(s) Martien van den Oever, Karin Molenveld, Maarten van der Zee, Harriëtte Bos Number Wageningen Food & Biobased Research number 1722 ISBN-number 978-94-6343-121-7 DOI http://dx.doi.org/10.18174/408350 Date of publication April 2017 Version Concept Confidentiality No/yes+date of expiration OPD code OPD code Approved by Christiaan Bolck Review Intern Name reviewer Christaan Bolck Sponsor RVO.nl + Dutch Ministry of Economic Affairs Client RVO.nl + Dutch Ministry of Economic Affairs Wageningen Food & Biobased Research P.O. Box 17 NL-6700 AA Wageningen Tel: +31 (0)317 480 084 E-mail: [email protected] Internet: www.wur.nl/foodandbiobased-research © Wageningen Food & Biobased Research, institute within the legal entity Stichting Wageningen Research All rights reserved. No part of this publication may be reproduced, stored in a retrieval system of any nature, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publisher. The publisher does not accept any liability for inaccuracies in this report. 2 © Wageningen Food & Biobased Research, institute within the legal entity Stichting Wageningen Research Preface For over 25 years Wageningen Food & Biobased Research (WFBR) is involved in research and development of bio-based materials and products. -
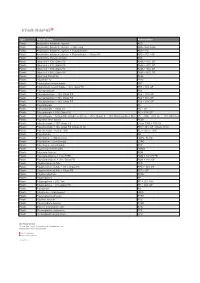
Type Material Name Abbreviation Plastic Acrylonitrile Butadiene
Type Material Name Abbreviation Plastic Acrylonitrile butadiene styrene ABS Plastic Acrylonitrile butadiene styrene - High-Temp ABS - high temp Plastic Acrylonitrile butadiene styrene + Polycarbonate ABS + PC Plastic Acrylonitrile butadiene styrene + Polycarbonate + Glass Fill ABS + PC + GF Plastic Acrylonitrile styrene acrylate ASA Plastic Nylon 6-6 + 10% Glass Fill PA66 + 10% GF Plastic Nylon 6-6 + 20% Glass Fill PA66 + 20% GF Plastic Nylon 6-6 + 30% Glass Fill PA66 + 30% GF Plastic Nylon 6-6 + 50% Glass Fill PA66 + 50% GF Plastic Nylon 6-6 Polyamide PA66 Plastic Polyamide 12 PA12 Plastic Polybutylene terephthalate PBT Plastic Polybutylene terephthalate + 30% Glass Fill PBT+ 30% GF Plastic Polycaprolactam PA6 Plastic Polycaprolactam + 20% Glass Fill PA6 + 20% GF Plastic Polycaprolactam + 30% Glass Fill PA6 + 30% GF Plastic Polycaprolactam + 50% Glass Fill PA6 + 50% GF Plastic Polycarbonate PC Plastic Polycarbonate + Glass Fill PC + GF Plastic Polycarbonate + 10% Glass Fill PC + 10% GF Plastic Polycarbonate + Acrylonitrile butadiene styrene + 20% Glass Fill + 10% Stainless Steel fiber PC + ABS + 20% GF + 10% SS Fiber Plastic Polyether ether ketone PEEK Plastic Polyetherimide + 30% Glass Fill Ultem 1000 + 30% GF Plastic Polyetherimide + 40% Glass Fill (Ultem 2410) PEI + 40% GF (Ultem 2410) Plastic Polyetherimide + Ultem 1000 PEI + Ultem 1000 Plastic Polyethylene PE Plastic Polyethylene - High-Density HDPE, PEHD Plastic Polyethylene - Low-Density LDPE Plastic Polyethylene terephthalate PET Plastic Polymethyl methacrylate PMMA Plastic Polyoxymethylene -

Environmental Impacts of Polyvinyl Chloride Building Materials
Environmental Impacts of Polyvinyl Chloride Building Materials by Joe Thornton, Ph.D. A Healthy Building Network Report Environmental Impacts of Polyvinyl Chloride Building Materials by Joe Thornton, Ph.D. A Healthy Building Network Report This report was prepared by the author and does not represent the opinions of The University of Oregon or any of its affiliates. © Healthy Buiding Network, 2002 Washington, D.C. The United States ISBN 0-9724632-0-8 Printed in the United States of America on acid-free, recycled paper, using soy-based ink. Photo Credits: p. 1 © Greenpeace p. 41 © Leuders/Greenpeace. p. 53 © Basel Action Network. PVC coated wire waste sorted and burned by villagers in Guiyu, China. Cancer causing polycyclic aro- matic hydrocarbons and dioxins will result from burning wires made from PVC and brominated flame retardants. p. 77 © Bryce lankard/Greenpeace 1996. Cows graze outside PVC manufacturing facility in Giesmar, Louisiana. Dioxin emissions to the environment move up the food chain. Fatty foods such as meat and dairy products are the primary source of dioxin exposure to most humans. p. 97 © Stone/Greenpeace 200. Lake Charles, Louisiana area children suffer health impacts due to pollution from PVC manufacturing facilities. Acknowledgements The Healthy Building acknowledges the following people and organiza- tions for their important contributions to this report. Dr. Joe Thornton’s willingness to accept this assignment on short notice and under a short deadline is testament to his ongoing commitment to the related goals