Polycarbonate (PC) Polysurfone (PSF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polycarbonate Lenses
Polycarbonate Lenses The most impact resistant of all lens materials is polycarbonate. Children, athletes, anyone working at a job or hobby where they might get hit in the face and need safety glasses, people with only one eye, those who fall a lot, are natural candidates for polycarbonate lenses. If safety is a prime concern, choose polycarbonate lenses. Advantages of polycarbonate lenses : 1. Polycarbonate has four to five times the impact resistance of glass or plastic. When glass or plastic lenses break, they do not break into harmless granules, but can break into sharp shards that can enter your eye and destroy your vision. Poly- carbonate is far and away the safest of all the lenses made. 2. Polycarbonate is the lightest lens material made. 3. Polycarbonate lenses naturally provide protection against ultra-violet light, at no additional charge. 4. Polycarbonate lenses come with a scratch resistant coating (not scratch proof) at no additional charge. 5. Polycarbonate is a high index material, so the lenses will be thinner than if made with glass or plastic. Disadvantages of polycarbonate lenses : 1. People in prescriptions with higher powers sometimes have trouble seeing out the edges of the lenses--your clear field of vision is not as wide as with glass or plas- tic lenses. The lenses are made with different curves than are used to make the same pre- scription power in glass or plastic, so you will see out of these lenses a little dif- ferently. People with prescriptions up to plus or minus three diopters (most people) usually have no problem adjusting to polycarbonate lenses. -

Development of Polysulfone Hollow Fiber Porous Supports for High Flux Composite Membranes: Air Plasma and Piranha Etching
fibers Article Development of Polysulfone Hollow Fiber Porous Supports for High Flux Composite Membranes: Air Plasma and Piranha Etching Ilya Borisov 1,*, Anna Ovcharova 1,*, Danila Bakhtin 1, Stepan Bazhenov 1, Alexey Volkov 1, Rustem Ibragimov 2, Rustem Gallyamov 2, Galina Bondarenko 1, Rais Mozhchil 3, Alexandr Bildyukevich 4 and Vladimir Volkov 1 1 A.V. Topchiev Institute of Petrochemical Synthesis, Russian Academy of Sciences, Moscow 119991, Russia; [email protected] (D.B.); [email protected] (S.B.); [email protected] (A.V.); [email protected] (G.B.); [email protected] (V.V.) 2 Kazan National Research Technological University, Kazan 420015, Russia; [email protected] (R.I.); [email protected] (R.G.) 3 National Research Nuclear University “MEPhI”, Moscow 115409, Russia; [email protected] 4 Institute of Physical Organic Chemistry, National Academy of Sciences of Belarus, Minsk 220072, Belarus; [email protected] * Correspondence: [email protected] (I.B.); [email protected] (A.O.); Tel.: +7-495-955-4893 (I.B.); +7-495-647-59-27 (A.O.) Academic Editors: Alberto Figoli and Tao He Received: 30 December 2016; Accepted: 4 February 2017; Published: 13 February 2017 Abstract: For the development of high efficiency porous supports for composite membrane preparation, polysulfone (PSf) hollow fiber membranes (outer diameter 1.57 mm, inner diameter 1.12 mm) were modified by air plasma using the low temperature plasma treatment pilot plant which is easily scalable to industrial level and the Piranha etch (H2O2 + H2SO4). Chemical and plasma modification affected only surface layers and did not cause PSf chemical structure change. -

Radel® PPSU, Udel® PSU, Veradel® PESU & Acudel® Modified PPSU
Radel ® | Udel ® | Veradel ® | Acudel ® Radel® PPSU, Udel® PSU, Veradel® PESU & Acudel® modified PPSU Processing Guide SPECIALT Y POLYMERS 2 \ Sulfone Polymers Processing Guide Table of Contents Introduction ............................. 5 Part Ejection . 14 Draft . 14 Ejector pins and/or stripper plates . 14 Sulfone Polymers........................ 5 Udel® Polysulfone (PPSU) . 5 Injection Molding Equipment ............. 15 ® Veradel Polyethersulfone (PESU) . 5 Controls . 15 ® Radel Polyphenylsulfone (PPSU) . 5 Clamp . 15 ® Acudel modified PPSU . 5 Barrel Capacity . 15 Press Maintenance . 15 Resin Drying . .6 Screw Design . 15 Rheology................................ 8 Screw Tips and Check Valves . 15 Viscosity-Shear Rate ..................... 8 Nozzles . 16 Molding Process . 16 Resin Flow Characteristics . 9 Melt flow index . 9 Polymer Injection or Mold Filling . 16 Spiral flow . 9 Packing and Holding . 17 Injection Molding . .10 Cooling . 17 Molds and Mold Design .................. 10 Machine Settings ....................... 17 Tool Steels . 10 Barrel Temperatures . 17 Mold Dimensions . 10 Mold Temperature . 18 Mold Polishing . 10 Residence Time in the Barrel . 18 Mold Plating and Surface Treatments . 10 Injection Rate . 18 Tool Wear . 10 Back Pressure . 18 Mold Temperature Control . 10 Screw Speed . 18 Mold Types . 11 Shrinkage . 18 Two-plate molds . 11 Three-plate molds . 11 Regrind ............................... 19 Hot runner molds . 11 Cavity Layout . 12 Measuring Residual Stress ............... 19 Runner Systems . 12 Extrusion............................... 22 Gating . 12 Sprue gating . 12 Edge gates . 13 Predrying ............................. 22 Diaphragm gates . 13 Tunnel or submarine gates . 13 Extrusion Temperatures ................. 22 Pin gates . 13 Screw Design Recommendations . 22 Gate location . 13 Venting . 14 Sulfone Polymers Processing Guide / 3 Die Design ............................. 22 Extruded Product Types . 23 Wire . 23 Film . 23 Sheet . 23 Piping and tubing . 23 Start-Up, Shut-Down, and Purging ....... -

Migration of Bisphenol a from Polycarbonate Plastic of Different Qualities
Migration of bisphenol A from polycarbonate plastic of different qualities Environmental project No. 1710, 2015 [Series Title and year] Title: Editing: Migration of Bisphenol A from polycarbonate Gitte Alsing Pedersen, DTU National Food Institute, plastic of different qualities Søren Hvilsted, DTU Danish Polymer Centre, Department of Chemical and Biochemical Engineering and Jens Højslev Petersen, DTU National Food Institute Technical University of Denmark Published by: The Danish Environmental Protection Agency Strandgade 29 1401 Copenhagen K Denmark www.mst.dk/english Year: ISBN no. 2015 978-87-93352-24-7 Disclaimer: When the occasion arises, the Danish Environmental Protection Agency will publish reports and papers concerning research and development projects within the environmental sector, financed by study grants provided by the Danish Environmental Protection Agency. It should be noted that such publications do not necessarily reflect the position or opinion of the Danish Environmental Protection Agency. However, publication does indicate that, in the opinion of the Danish Environmental Protection Agency, the content represents an important contribution to the debate surrounding Danish environmental policy. Sources must be acknowledged. 2 Migration of Bisphenol A from polycarbonate plastic of different qualities Contents Foreword .................................................................................................................. 5 Conclusion and Summary ......................................................................................... -

Blends of Polycarbonate Containing Fluorinated-Bisphenol-A and Polyvinyl Chloride
Europaisches Patentamt European Patent Office © Publication number: 0 576 057 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 93201533.2 int. Ci.5; C08L 69/00, C08L 27/06, C08G 64/10, //(C08L69/00, @ Date of filing: 28.05.93 27:06),(C08L27/06,69:00) © Priority: 01.06.92 US 891032 © Applicant: ENICHEM S.p.A. Piazza della Repubblica, 16 @ Date of publication of application: 1-20124 Milano(IT) 29.12.93 Bulletin 93/52 @ Inventor: Drzewinski, Michael A. © Designated Contracting States: 371 Clarksville Road, Princeton Junction AT BE CH DE DK ES FR GB GR IE IT LI LU MC New Jersey 08850(US) NL PT SE © Representative: Roggero, Sergio et al Ing. Barzano & Zanardo Milano S.p.A. Via Borgonuovo 10 1-20121 Milano (IT) © Blends of polycarbonate containing fluorinated-bisphenol-A and polyvinyl chloride. © Bisphenol A polycarbonate containing at least 15 mole % of 2,2-bis-(4-hydroxyphenyl)hexafluoropropane (6F-Bisphenol A) can be blended with polyvinyl chloride (PVC) to form a thermodynamically miscible, transpar- ent, single phase blend at all compositions. Such blends are flame resistant as well as resistant to attack by acids, bases and many organic solvents. CO Rank Xerox (UK) Business Services (3. 10/3.6/3.3. 1) EP 0 576 057 A1 BACKGROUND OF THE INVENTION Field of the Invention: 5 This invention pertains to mixtures of polyvinyl chloride (PVC) and polycarbonates which contain at least 15 mole % of fluorinated bisphenol monomer units (F-PC) such as 2,2-bis-(4-hydroxyphenyl)- hexafluoropropane (6F-bisphenol A), herein referred to as 6F-PC. -

Technical Datasheet: Udel® P-1700
Udel® P-1700 Polysulfone Solvay Specialty Polymers www.ulprospector.com Technical Data Product Description Udel® P-1700 polysulfone (PSU) is a tough, rigid, high-strength thermoplastics suitable for continuous use up to 300°F (149°C). It is resistant to oxidation and hydrolysis and withstand prolonged exposure to high temperatures and repeated sterilization. Udel® P-1700 polysulfone is highly resistant to mineral acids, alkali and salt solutions. Resistance to detergents and hydrocarbon oils is good, but the resin may be attacked by polar solvents such as ketones, chlorinated hydrocarbons and aromatic hydrocarbons. These resins are also highly resistant to degradation by gamma or electron beam radiation. Electrical properties of Udel® P-1700 polysulfones are stable over a wide temperature range and after immersion in water or exposure to high humidity. The resins comply with FDA 21 CFR 177.1655 and may be used in articles intended for repeated use in contact with foods. Additionally, they are approved by the NSF, by the Department of Agriculture for contact with meat and poultry and by the 3-A Sanitary Standards of the Dairy Association. • Transparent: Udel® P-1700 CL 2611 CMP • Transparent: Udel® P-1700 NT 06 • Transparent: Udel® P-1700 NT 11 • Opaque Black : Udel® P-1700 BK 937 • Opaque White: Udel® P-1700 WH 6417 • Opaque White: Udel® P-1700 WH 7407 • Opaque Gray: Udel® P-1700 GY 8057 General Material Status • Commercial: Active Literature 1 • Technical Datasheet UL Yellow Card 2 • E36098-231084 • Solvay Specialty Polymers Search for -

Studies on Radiation Crosslinking of Polysulfone
JP0050427 JAERI-Conf 2000-001 Studies on Radiation Crosslinking of Polysulfone Xiaoguang Zhong Jiazhen Sun Changchun Institute of Applied Chemistry Chinese Academy of Sciences Changchun 130022, China Polysulfone is a kind of high temperature-resistance and radiation-resistance engineering plastic. The chemical structure is as follows: Brown (1), Lyon (2), Sasuga (3), et al have already studied its radiation effect. We studied CH3 radiation crosslinking effect of polysulfone by using of XPS, ESR, and CG methods and got some new results. Results and Discussions 1. Study radiation crosslinking of polysulfone by XPS method Because of conjugate system of benzene ring, polymer material which contains of benzene ring will appear shake-up peak in XPS spectra. Wanxi Zhang (4) shows that during radiation crosslinking of polystyrene increases with radiation dose and the intensity of shake-up peak decreases gradually with increase of radiation dose and crosslinking degree. This suggests that radiation crosslinking destroyed conjugate system of benzene ring. During radiation crosslinking of polysulfone, we find rules of shake-up peaks in the XPS spectra are different at different radiation crosslinking temperature. At lower temperature the intensity of shake-up peak decreases with the increase of radiation dose. This rule is similar to that of radiation crosslinking of polystyrene. The results are shown in Fig. 1. 183- JAERI-Conf 2000-001 Fig. 1. The spectra of radiation crosslinking of polysulfone at 70"C Comparing with radiation crosslinking at lower temperature, the intensity of shake-up peak increases with radiation dose when radiation crosslinking reaction takes place at temperature above glass transition temperature of polysulfone. -

Study of Surface Mechanical Characteristics of ABS/PC Blends Using Nanoindentation
processes Article Study of Surface Mechanical Characteristics of ABS/PC Blends Using Nanoindentation Saira Bano 1, Tanveer Iqbal 2, Naveed Ramzan 3 and Ujala Farooq 4,* 1 Department of Chemical & Polymer Engineering, University of Engineering & Technology, FSD Campus, Lahore 38000, Pakistan; [email protected] 2 Department of Chemical, Polymer & Composite Materials Engineering, University of Engineering & Technology, KSK Campus, Lahore 54890, Pakistan; [email protected] 3 Department of Chemical Engineering, University of Engineering & Technology, KSK Campus, Lahore 54890, Pakistan; [email protected] 4 Faculty of Aerospace Engineering, Aerospace Manufacturing Technologies, Delft University of Technology, Kluyverweg 1, 2629 HS Delft, The Netherlands * Correspondence: [email protected] Abstract: Acrylonitrile butadiene styrene (ABS) and polycarbonate (PC) are considered a well-known class of engineering thermoplastics due to their efficient use in automotive, 3D printing, and elec- tronics. However, improvement in toughness, processability, and thermal stability is achieved by mixing together ABS and PC. The present study focuses on the understanding of surface mechani- cal characterization of acrylonitrile butadiene styrene (ABS) and polycarbonate (PC) blends using nano-indentation. Polymer blends sheets with three different proportions of ABS/PC (75:25, 50:50, and 25:75) were fabricated via melt-processing and thermal press. Fourier transform infrared (FTIR) spectroscopy was performed to analyze the intermolecular interactions between the blends’ compo- nents. To understand the surface mechanical properties of ABS and PC blends, a sufficient number Citation: Bano, S.; Iqbal, T.; Ramzan, of nano-indentation tests were performed at a constant loading rate to a maximum load of 100 mN. N.; Farooq, U. -

US3636140.Pdf
Jan. 18, 1972 A. F. INGULLI ETA 3,636,140 THERMOPLASTIC RESIN BLEND OF POLY SULFONE WITH ABS Filed Aug. 4, 1969 5. Sheets-Sheet, 2. R O /O 20 30 40 0 60 22 80 20 M22 (7, AOA. Yuo/MAOM/A /W AA S adze wa /MVA MV7 OAS Air 7- 4. a Zafa AA A. MM/G/ZZ / A/a. Mary A. at 7A air 2- 9% agew, Jan. 18, 1972 A. F. NGULL ET All- 3,636,140 "I HERMOPLASTIC RESIN BLEND OF POLYSULFONE WITH ABS Filed Aug. 4, 1969 5. Sheets-Sheet 3 S | | | | | | \ / | | | | |\ 1 \ | | | | | | | | \ | | | | | | | N TTTTTTTT O 3O 20 O SO 70 so 90 /OO 7. AozYuva /owa w Aaj azawo 2g z 7-5 MM/a M72A S. AAAAAA M. MMMGO/4 AM AyAAMA 1 A... a A. JAA larus 9.4 AG AW 7 Jan. 18, 1972 A. F. NGULL FT All- 3,636,140 TERMOPLASTIC RESIN BLEND OF POLYSULFONE WITH ABS Filed Aug. 4, 1969 5 Sheets-Sheet 4. 24.0 -- -T 22.ol | | | || 20.0 I Y Mas t SR I W. n w /4.0 | -- N - S v 2.0 HI N | n N S s S. v O /O 20 30 10 60 60 70 &0 70 /00 7./oz ruz/z/-owa //v4 as aza/v4 MM VAM/OA 27zz7 a AAAMAA A. MM6t/44/ A/AMMA)1 A. az Zafa 9ers /.4- a 47AM/7 Jan. 18, 1972 A. F. NGULL ET All- 3,636,140 THERMOPLASTIC RESIN BLEND OF POLYSULFONE WITH ABS Filed Aug. 4, 1969 5 Sheets-Sheet 5 JO J00 290 28 O 270 28 O 25 O 24 O 2.3 O O O 20 JO 40 50 60 70 8 O 3 O LOO % AOZyura/4 MoM2 //v 44, aza/V2 , , , . -

High Performance Reactive Blends Composed of Poly(P-Phenylene Sulfide)
Polymer Journal (2011) 43, 991–999 & The Society of Polymer Science, Japan (SPSJ) All rights reserved 0032-3896/11 $32.00 www.nature.com/pj ORIGINAL ARTICLE High performance reactive blends composed of poly(p-phenylene sulfide) and ethylene copolymers Hideko T Oyama, Mayu Matsushita and Motonobu Furuta Poly(p-phenylene sulfide) (PPS) is a high performance polymer that has superior chemical resistance and heat stability, but its brittleness is a serious drawback for applications. The objective of this work is to improve the physical properties of PPS by incorporating a small amount of either poly(ethylene-ran-methylacrylate–ran-glycidyl methacrylate) (EMA–GMA) or poly(ethylene- ran-glycidyl methacrylate)-graft-poly(methyl methacrylate) (EGMA-g-PMMA) by melt mixing under a high shear rate. It was demonstrated that the chemical reaction between PPS and EMA–GMA (or EGMA-g-PMMA) proceeded efficiently at the interface and that the domains of EMA–GMA (or EGMA-g-PMMA) were finely dispersed in the PSS matrix with size of ca 0.1–0.3 lm. The resultant copolymers formed at the interface contributed to a decrease in the interfacial tension and an increase in the interfacial adhesion so that the obtained PPS/EMA–GMA blends (or PPS/EGMA-g-PMMA blends) showed excellent mechanical properties, at the same time retaining high thermal stability. Polymer Journal (2011) 43, 991–999; doi:10.1038/pj.2011.106; published online 19 October 2011 Keywords: blend; copolymer; interface; poly(ethylene-ran-glycidyl methacrylate); poly(phenylene sulfide) INTRODUCTION ties are not reported in the same paper, it was found that the Poly(phenylene sulfide) (PPS) is a high performance super-engineer- activation energy of crystallization of PPS increases by blending ing plastic with high thermal stability (over 150 1C), excellent chemical with PES and that the equilibrium melting temperature decreases resistance (no solvents under 200 1C), good electrical and electronic linearly with increase of the PES content. -
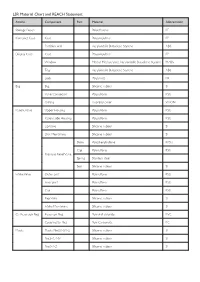
LSR Material Chart and REACH Statement
LSR Material Chart and REACH Statement Article Component Part Material Abbreviation Storage Pouch Polyethylene PE Compact Case Case Polypropylene PP Partition wall Acrylonitrile Butadiene Styrene ABS Display Case Case Polypropylene PP Window Methyl Methacrylate Acrylonitrile Butadiene Styrene MABS Tray Acrylonitrile Butadiene Styrene ABS Lock Polyamide PA Bag Bag Silicone rubber SI Valve Connector Polysulfone PSU O-Ring Fluorelastomer VITON Patient Valve Upper Housing Polysulfone PSU Patient side Housing Polysulfone PSU Lip Valve Silicone rubber SI Disk Membrane Silicone rubber SI Stem Polyphenylsulfone PPSU Cap Polysulfone PSU Pressure Relief Valve Spring Stainless steel Seal Silicone rubber SI Intake Valve Outer part Polysulfone PSU Inner part Polysulfone PSU Cap Polysulfone PSU Flap Valve Silicone rubber SI Intake Membrane Silicone rubber SI O² Reservoir Bag Reservoir Bag Polyvinyl chloride PVC Coupling for Bag Poly Carbonate PC Masks Masks No.00-0/1-2 Silicone rubber SI No.3-4, 4-5+ Silicone rubber SI No.0-1-2 Silicone rubber SI Article Component Part Material Abbreviation Mask Cover Polysulfone PSU Lock Clip Stainless steel Head Strap w/Ring Strap Thermoplastic Elastomer TPE Attachment Ring Polysulfone PSU Expiration Diverter Housing Polysulfone PSU Center gasket Silicone rubber SI External gasket Silicone rubber SI Extension Tube Tube Silicone rubber SI Coupling Polysulfone PSU Manometer Polysulfone PSU Connector Hanging Loop Silicone rubber SI Wall Mount Acrylonitrile Butadiene Styrene ABS Wall Bracket PolyOxyMethylene POM Laerdal Statement for REACH Regulation EC No 1907/2006 Substances of Very High Concern are not used in concentrations above 0.1% (w/w) in the production of all models of the Laerdal Silicone Resuscitator. -

Polysulfone PSU1000 Datasheet
Quality Plastics Since 1936 POLYSULFONE - PSU1000 Characteristics * Broad Temperature Range Capability - Hot Water and Steam Performance to 300º F (150 C) * Good Thermal and Electrical Insulation Characteristics * Hydrolysis Resistant * Radiation Stability * Low Ionic Impurity Description PSU 1000 Polysulfone is an amber semi-transparent, heat resistant, high performance engineering thermoplastic. It offers excellent mechanical, electrical and improved chemical resistance properties relative to polycarbonate. Polysulfone's properties remain relatively consistent over a broad temperature range, from -150º to 300º F (-100º to 150º C). PSU Polysulfone offers high chemical resistance to acidic and salt solutions, and good resistance to detergents, hot water and steam. In addition, polysulfone has excellent radiation stability and offers low ionic impurity levels. PSU 1000 Polysulfone often replaces polycarbonate when higher temperatures, improved chemical resistance or autoclavability is required. It is commonly used for analytical instrumentation, medical devices and semiconductor process equipment components. Forms Available ROD .250 to 6.00 " diameter, Lengths to 12 feet TUBE Custom produced as requested PLATE .250" to 3.000" thick, Widths to 24", Lengths to 48" COLOR Amber Typical Property Values Polysulfone MECHANICAL @ 73ºF PSU-1000 Specific Gravity 1.24 Tensile Strength psi 10,200 Tensile Modulus of Elasticity psi 390,000 Tensile Elongation ( at Break ) % 30 Flexural Strength psi 15,000 Flexural Modulus of Elasticity psi 400,000 Shear Strength psi 9,000 Compressive Strength, 10% Deformation psi 13,000 Compressive Modulus of Elasticity psi 375,000 Rockwell Hardness M Scale 82 Izod Impact Strength, Notched ft-lbs/in. of notch 1.30 Coefficient of Friction, Dynamic ( Dry vs. Steel ) Limiting PV ( 4 :1 Safety Factor Applied ) ft.lbs/in.² min Wear Factor in³-min/ft.lbs.