Polycarbonate (PC)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polycarbonate Lenses
Polycarbonate Lenses The most impact resistant of all lens materials is polycarbonate. Children, athletes, anyone working at a job or hobby where they might get hit in the face and need safety glasses, people with only one eye, those who fall a lot, are natural candidates for polycarbonate lenses. If safety is a prime concern, choose polycarbonate lenses. Advantages of polycarbonate lenses : 1. Polycarbonate has four to five times the impact resistance of glass or plastic. When glass or plastic lenses break, they do not break into harmless granules, but can break into sharp shards that can enter your eye and destroy your vision. Poly- carbonate is far and away the safest of all the lenses made. 2. Polycarbonate is the lightest lens material made. 3. Polycarbonate lenses naturally provide protection against ultra-violet light, at no additional charge. 4. Polycarbonate lenses come with a scratch resistant coating (not scratch proof) at no additional charge. 5. Polycarbonate is a high index material, so the lenses will be thinner than if made with glass or plastic. Disadvantages of polycarbonate lenses : 1. People in prescriptions with higher powers sometimes have trouble seeing out the edges of the lenses--your clear field of vision is not as wide as with glass or plas- tic lenses. The lenses are made with different curves than are used to make the same pre- scription power in glass or plastic, so you will see out of these lenses a little dif- ferently. People with prescriptions up to plus or minus three diopters (most people) usually have no problem adjusting to polycarbonate lenses. -

Migration of Bisphenol a from Polycarbonate Plastic of Different Qualities
Migration of bisphenol A from polycarbonate plastic of different qualities Environmental project No. 1710, 2015 [Series Title and year] Title: Editing: Migration of Bisphenol A from polycarbonate Gitte Alsing Pedersen, DTU National Food Institute, plastic of different qualities Søren Hvilsted, DTU Danish Polymer Centre, Department of Chemical and Biochemical Engineering and Jens Højslev Petersen, DTU National Food Institute Technical University of Denmark Published by: The Danish Environmental Protection Agency Strandgade 29 1401 Copenhagen K Denmark www.mst.dk/english Year: ISBN no. 2015 978-87-93352-24-7 Disclaimer: When the occasion arises, the Danish Environmental Protection Agency will publish reports and papers concerning research and development projects within the environmental sector, financed by study grants provided by the Danish Environmental Protection Agency. It should be noted that such publications do not necessarily reflect the position or opinion of the Danish Environmental Protection Agency. However, publication does indicate that, in the opinion of the Danish Environmental Protection Agency, the content represents an important contribution to the debate surrounding Danish environmental policy. Sources must be acknowledged. 2 Migration of Bisphenol A from polycarbonate plastic of different qualities Contents Foreword .................................................................................................................. 5 Conclusion and Summary ......................................................................................... -

Blends of Polycarbonate Containing Fluorinated-Bisphenol-A and Polyvinyl Chloride
Europaisches Patentamt European Patent Office © Publication number: 0 576 057 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 93201533.2 int. Ci.5; C08L 69/00, C08L 27/06, C08G 64/10, //(C08L69/00, @ Date of filing: 28.05.93 27:06),(C08L27/06,69:00) © Priority: 01.06.92 US 891032 © Applicant: ENICHEM S.p.A. Piazza della Repubblica, 16 @ Date of publication of application: 1-20124 Milano(IT) 29.12.93 Bulletin 93/52 @ Inventor: Drzewinski, Michael A. © Designated Contracting States: 371 Clarksville Road, Princeton Junction AT BE CH DE DK ES FR GB GR IE IT LI LU MC New Jersey 08850(US) NL PT SE © Representative: Roggero, Sergio et al Ing. Barzano & Zanardo Milano S.p.A. Via Borgonuovo 10 1-20121 Milano (IT) © Blends of polycarbonate containing fluorinated-bisphenol-A and polyvinyl chloride. © Bisphenol A polycarbonate containing at least 15 mole % of 2,2-bis-(4-hydroxyphenyl)hexafluoropropane (6F-Bisphenol A) can be blended with polyvinyl chloride (PVC) to form a thermodynamically miscible, transpar- ent, single phase blend at all compositions. Such blends are flame resistant as well as resistant to attack by acids, bases and many organic solvents. CO Rank Xerox (UK) Business Services (3. 10/3.6/3.3. 1) EP 0 576 057 A1 BACKGROUND OF THE INVENTION Field of the Invention: 5 This invention pertains to mixtures of polyvinyl chloride (PVC) and polycarbonates which contain at least 15 mole % of fluorinated bisphenol monomer units (F-PC) such as 2,2-bis-(4-hydroxyphenyl)- hexafluoropropane (6F-bisphenol A), herein referred to as 6F-PC. -

Study of Surface Mechanical Characteristics of ABS/PC Blends Using Nanoindentation
processes Article Study of Surface Mechanical Characteristics of ABS/PC Blends Using Nanoindentation Saira Bano 1, Tanveer Iqbal 2, Naveed Ramzan 3 and Ujala Farooq 4,* 1 Department of Chemical & Polymer Engineering, University of Engineering & Technology, FSD Campus, Lahore 38000, Pakistan; [email protected] 2 Department of Chemical, Polymer & Composite Materials Engineering, University of Engineering & Technology, KSK Campus, Lahore 54890, Pakistan; [email protected] 3 Department of Chemical Engineering, University of Engineering & Technology, KSK Campus, Lahore 54890, Pakistan; [email protected] 4 Faculty of Aerospace Engineering, Aerospace Manufacturing Technologies, Delft University of Technology, Kluyverweg 1, 2629 HS Delft, The Netherlands * Correspondence: [email protected] Abstract: Acrylonitrile butadiene styrene (ABS) and polycarbonate (PC) are considered a well-known class of engineering thermoplastics due to their efficient use in automotive, 3D printing, and elec- tronics. However, improvement in toughness, processability, and thermal stability is achieved by mixing together ABS and PC. The present study focuses on the understanding of surface mechani- cal characterization of acrylonitrile butadiene styrene (ABS) and polycarbonate (PC) blends using nano-indentation. Polymer blends sheets with three different proportions of ABS/PC (75:25, 50:50, and 25:75) were fabricated via melt-processing and thermal press. Fourier transform infrared (FTIR) spectroscopy was performed to analyze the intermolecular interactions between the blends’ compo- nents. To understand the surface mechanical properties of ABS and PC blends, a sufficient number Citation: Bano, S.; Iqbal, T.; Ramzan, of nano-indentation tests were performed at a constant loading rate to a maximum load of 100 mN. N.; Farooq, U. -
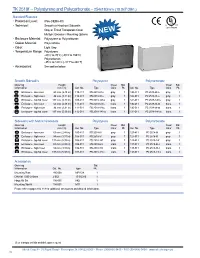
TK 2518F – Polystyrene and Polycarbonate – 254X180mm (10.0X7.09In.)
TK 2518f – Polystyrene and Polycarbonate – 254x180mm (10.0x7.09in.) Standard Features • Protection Level: IP66 (NEMA 4X) • Technical: Smooth or Knockout Sidewalls Upon Request Gray or Tinted Transparent Cover ➀ Multiple Enclosure Mounting Options NEW • Enclosure Material: Polystyrene or Polycarbonate • Gasket Material: Polyurethane • Color: Light Gray • Temperature Range: Polystyrene -40°C to 70°C (-40°F to 158°F) Polycarbonate -35°C to 120°C (-31°F to 248°F) • Accessories: See section below Smooth Sidewalls Polystyrene Polycarbonate Ordering Height Cover Std. Cover Std. Information mm (in) Cat. No. Type Color Pk. Cat. No. Type Color Pk. Enclosure - low cover 63 mm (2.48 in) 110-411 PS 2518-6f-o gray 1 120-411 PC 2518-6f-o gray 1 Enclosure - high cover 84 mm (3.31 in) 110-911 PS 2518-8f-o gray 1 120-911 PC 2518-8f-o gray 1 Enclosure - top hat cover 137 mm (5.39 in) 109-411 PS 2518-14f-o gray 1 121-411 PC 2518-14f-o gray 1 Enclosure - low cover 63 mm (2.48 in) 111-011 PS 2518-6f-to trans. 1 130-011 PC 2518-6f-to trans. 1 Enclosure - high cover 84 mm (3.31 in) 111-511 PS 2518-8f-to trans. 1 130-511 PC 2518-8f-to trans. 1 Enclosure - top hat cover 137 mm (5.39 in) 112-011 PS 2518-14f-to trans. 1 131-011 PC 2518-14f-to trans. 1 Sidewalls with Metric Knockouts Polystyrene Polycarbonate Ordering Height Cover Std. Cover Std. Information mm (in) Cat. No. Type Color Pk. -

PVC (Polyvinyl Chloride)~ FACT SHEET & Talking Points
PVC (Polyvinyl Chloride)~ FACT SHEET & Talking Points http://archive.greenpeace.org/toxics/html/contenUpvc1.html http://www.greenpeace.org/usa/en/campaigns/toxics/go-pvc-free/ http://www.who.inUmediacentre/factsheets/fs225/en/ http://www.greenpeace.org/usa/en/campaigns/!oxics/go-pvc-free/ http://www.ens-newswire.com/ens/jan2011/2011-01-21-01.html What is PVC? >- The most environmentally damaging plastic. In fact, this commonplace plastic is one of the most toxic substances saturating our planet and its inhabitants >- From cradle to grave, the PVC Lifecycle (production, use and disposal) results in the release of toxic, chlorine based chemicals. P:. One of the world's largest dioxin sources ~ These toxins build up in water, air and in the food chain. ;... The results: severe health problems, including cancer, immune system damage and hormone disruption. ;... No one can escape contamination. P:. Everyone everywhere has measureable levels of chlorinated toxins in their bodies. How can we get rid of PVC? >- Since safer alternatives are available for virtually all uses of PVC, it is possible to protect human health and the environment by replacing and eventually phasing out this poison plastic. >- Policymakers at the local, state and federal level should enact and implement laws that steadily reduce impacts of PVC disposal and lead to a complete phase out of PVC use and waste incineration within ten years. >- A new materials policy for PVC that embraces aggressive source reduction of PVC should be adopted to steadily reduce the use of PVC over time liPage Background Information "Due to the omnipresence of dioxins, all people have background exposure and a certain level of dioxins in the body, leading to the so-called body burden. -

Smart Plastics Guide Healthier Food Uses of Plastics
INSTITUTE FOR AGRICULTURE AND TRADE POLICY < FOOD AND HEALTH PROGRAM Smart Plastics Guide Healthier Food Uses of Plastics Plastics are widely used to store and package food and beverages. What plastic labels mean: The recycling symbol is used pri- They are convenient, lightweight, unbreakable and relatively marily on disposable plastic packaging and single use contain- inexpensive. However, there are both environmental and health ers. Non-disposable food-use goods like dinnerware, pitchers, risks from the widespread use of plastics. flatware and baby bottles usually do not have a recycling label. NOTE: Not all containers are labeled, and a recycling symbol Environmental problems: Most plastics are made on a product doesn’t mean it’s recyclable. Commonly, only from petroleum, a non-renewable resource. Plastic packag- plastic products labeled #1 and #2 with narrow necks are re- ing also creates unnecessary waste. Plastic is bulky—taking cyclable, but some communities recycle other plastics. Check up a large volume of landfill space. with your local municipality or waste disposal company. Health risks: The use of plastics in cooking and food storage can carry health risks, especially when hormone- disrupting chemicals from some plastics leach into foods and PETE: Polyethylene terephthalate ethylene, used for beverages. Plastic manufacturing and incineration creates air soft drink, juice, water, detergent, cleaner and peanut and water pollution, and exposes workers to toxic chemicals. butter containers. HDPE: High density polyethylene, used in opaque plastic milk and water jugs, bleach, detergent and Choose less polluting products to reduce shampoo bottles, and some plastic bags. your exposure to chemicals. PVC or V: Polyvinyl chloride, used for cling wrap, plastic squeeze bottles, cooking oil and peanut butter PVC—THE POISON PLASTIC: Polyvinyl chloride, also containers, and detergent and window cleaner bottles. -

Gasoline Diffusivity of Polypropylene-Polycarbonate Composites
International Journal of Innovative Technology and Exploring Engineering (IJITEE) ISSN: 2278-3075, Volume-3, Issue-8, January 2014 Gasoline Diffusivity of Polypropylene-Polycarbonate Composites Buthainah A., Dayang Radiah A. B., Alnuami W., Ethar Y. Salih, Mohammed. A. Jawad Abstract- This manuscript explores the effect of polypropylene- The excellent fatigue resistance of PP is utilized in the polycarbonate composite (PP-PC) on gasoline absorption. moulding of integral hinges (e.g. accelerator pedals and Composites were prepared from PP-PC of different ratios with forceps/tweezers). and without carbon black as a stabilizer. Further, the effect of Polypropylene is also available in fiber form (for ropes, aging gasoline in the presence of carbon black for a period of two carpet backing) and as a film (for packaging) [7]. months was studied. A single screw extruder was used to produce Polycarbonate, on the other hand, is an engineering plastic these composites and form them into 2mm thick sheets. A that has many advantages over conventional materials in hydraulic compressor was then used to produce a 4mm sheet. Absorption test was carried out in gasoline at different immersion terms of mechanical and physical properties. Including times and different blends. The results showed that the extreme toughness. These plastics are diaphanous and have polycarbonate performance was improved upon addition of good temperature resistance but are attacked by alkaline polypropylene. The results of absorption show that it obey Fick's solutions and hydrocarbon solvents. Typical applications second law of diffusion and after the addition of carbon black the include vandal-proof street lamp covers, baby bottles, absorption decrease. -

Lexan 9030 Polycarbonate
GE Plastics Specialty Film & Sheet Lexan* 9030 Sheet Product Datasheet Description Lexan* 9030 sheet is the standard grade of Lexan sheet without UV protected nor Mar resistant surface treatment. Lexan 9030 sheet combines high impact and temperature resistance with optical clarity and can be utilized for secondary glazing behind existing glazing for economical protection against breakage or intrusion. Lexan 9030 sheet can be cut, sawn, drill, milling and bent easily using standard workshop equipment without the risk of cracking and breakage and is therefore an excellent candidate for fabricating a wide range of indoor applications such as machine guards etc. Lexan 9030 sheet can be easily thermoformed into complex parts while retaining its excellent properties necessary for demanding applications such as vandal proof street furniture. Lexan 9030 sheet may be decorated using a wide variety of modern techniques such as painting and screen printing. Typical Property Values Property Test Method Unit Value Physical Density ISO 1183 g/cm³ 1.2 Water absorption, 24 hours ISO 62 mg. 10 Water absorption, saturation /23°C ISO 62 % 0.35 Mould shrinkage ASTM-D955 % 0.6-0.8 Poison’s ratio ASTM-D638 - 0.38 Mechanical Tensile stress at yield 50 mm/min ISO 527 MPa 60 Tensile stress at break 50 mm/min ISO 527 MPa 70 Tensile strain at yield 50 mm/min ISO 527 % 6 Tensile strain at break 50 mm/min ISO 527 % 120 Tensile modulus 1 mm/min ISO 527 MPa 2350 Flexural stress at yield 2 mm/min ISO 178 MPa 90 Flexural modulus 2 mm/min ISO 178 MPa 2300 Hardness H358/30 95 ISO 2039/1 MPa 95 Impact Charpyimpact, notched ISO 179/2C kJ/m² 35 Izodimpact,unnotched23°C ISO 180/1U kJ/m² NB Izodimpact,unnotched-30°C ISO 180/1U kJ/m² NB Izod impact, notched 23°C ISO 180/1A kJ/m² 65 Izodimpact, notched -30°C ISO 180/1A kJ/m² 10 These property values have been derived from Lexan* resin data for the material used to produce this sheet product. -

Polycarbonate (PC) Polysurfone (PSF)
POLYMER MODIFICATION BY IMPROVED IRRADIATION TECHNOLOGY T. Seguchi, T. Yagi, S. Ishikawa*, and Y. Sano* MY0001468 Takasaki Radiation Chemistry Research Establishment, Japan Atomic Energy Research Institute, Takasaki, Gunma, 370-1292 Japan, Fax: (81) 27-346-9687, E-mail: [email protected] *The Shiyuukai Foundation, The Medical Machinery and Materials Laboratory, Masago, Niigata 950- 2074 Japan, Fax:(81) 25-233-8041 1. INTRODUCTION Irradiation technology for polymer modification has been widely applied; the processing of heat resistant wire and cables, polymer form, shrinkable tube, and so on. These applications are based on the radiation induced crosslinking among polymer chains, and the most of processing has been carried out at ambient temperature in atmosphere. It is known that the radiation induced chemical reactions are affected by the irradiation conditions such as atmosphere, temperature, but those effects are not so considerable problem on the processing for above applications. Recently, it was found that the chemical reactions are much dependent on the irradiation temperature for some polymers. A typical one is polytetrafluoroethylene(PTFE), which is chain scission by irradiation at ambient temperature, but crosslinking at the high temperature as the molten state(1). Another case is polystyrene(PS), which is crosslinking at ambient temperature, but chain scission become predominant above the glass transition temperature®. For engineering plastics as polycarbonate(PC) and polysulfone(PSF), the irradiation effects were widely studied on the point of irradiation temperature. A remarkable change on hardness for PC and PSF was observed at high temperature irradiation. This is a new technology for polymer modification by radiation application. 2. -

Surface Localization of Poly(Methyl Methacrylate) in a Miscible Blend with Polycarbonate
Polymer Journal (2015) 47, 576–579 & 2015 The Society of Polymer Science, Japan (SPSJ) All rights reserved 0032-3896/15 www.nature.com/pj ORIGINAL ARTICLE Surface localization of poly(methyl methacrylate) in a miscible blend with polycarbonate Takumi Sako, Shogo Nobukawa and Masayuki Yamaguchi A new method to localize poly(methyl methacrylate) (PMMA) at the surface of a miscible blend with polycarbonate (PC) is demonstrated. Low-molecular-weight PMMA, which is found to be miscible with PC, is used in this study. After annealing the PC/PMMA blend in a temperature gradient, PMMA is found to localize on the high temperature side, as detected by infrared spectroscopy and molecular weight measurements. Furthermore, the sample exhibits good transparency even after annealing. This phenomenon is notable because it is applicable to enhancing the anti-scratch properties of PC. Polymer Journal (2015) 47, 576–579; doi:10.1038/pj.2015.37; published online 10 June 2015 INTRODUCTION EXPERIMENTAL PROCEDURE Polycarbonate (PC) is widely employed in industrial applications, such Materials and sample preparation as plastic glasses, optical discs and exterior cases for electronic A commercially available bisphenol A PC (Panlite L-1225Y, Teijin, Osaka, equipment, because of its excellent transparency and mechanical Japan) was used in this study. Furthermore, two types of PMMA with different toughness. In the automobile industry, PC is expected to be used molecular weights were employed: a commercially available grade, H-PMMA rather than inorganic glass in future vehicles because of its light (Acrypet VH, Mitsubishi Rayon, Tokyo, Japan) and a second type kindly weight. provided by Mitsubishi Rayon (L-PMMA). -

An Example of the Use of Standard Flammability Criteria for Performance Analysis of Materials: Polycarbonate and PMMA
An Example of the Use of Standard Flammability Criteria for Performance Analysis of Materials: Polycarbonate and PMMA L. AUDOUIN1, J. L. TORERO2, O. MÅNGS3, L. RIGOLLET1 1 Institut de Radioprotection et de Sûreté Nucléaire/DPAM Bâtiment 702 -CE Cadarache, BP 3 13115 Saint Paul lez Durance, France 2 Edinburgh Centre for Fire Research, The University of Edinburgh Edinburgh, EH9 3JL, United Kingdom 3 Brandskyddslaget, Hornsbruksgatan 28 Box 9196 SE-102 73 Stockholm, Sweden ABSTRACT A detailed flammability evaluation of two different materials is presented, as an example of how important is physical observation and detailed analysis in the interpretation of test data. The objective is to provide different criteria that could allow the use of standard test data in fire modeling. The tests used are the lateral ignition and flame spread test (ASTM- E-1321) and the oxygen consumption calorimeter (ASTM-E-1354). The materials evaluated are a polycarbonate (Lexan) and compared to a commonly studied ideal material, PMMA (i.e., Poly-methyl-methacrylate). Lexan is a solid plastic sheet, generally considered as fire resistant. The solid plastic sheet is proposed as an alternate to PMMA for use as containment windows for glove boxes containing radioactive components in nuclear facilities. For idealized materials, such as PMMA, heat transfer from the flame to the fuel controls flame-spread rates. This study shows that the behavior of the Lexan, in the case of a fire, will be first governed by melting. Furthermore, if the material is placed vertical, the results indicate that the motion of the molten fuel, rather than the transport of energy will control flame-spread rates.