(12) Patent Application Publication (10) Pub. No.: US 2014/0350127 A1 CANO Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Iris for the Home Gardener a Rainbow of Colors in Many Shapes and Sizes Bob Lyons
Iris for the Home Gardener A Rainbow of Colors in Many Shapes and Sizes Bob Lyons FEW PLANTS HAVE AS MUCH HISTORY and affection among gardeners than iris. In Greek mythology, Iris is the personification of the rainbow and messenger of the Gods, and indeed, Iris appear in many magical colors—a large and diverse genus. Some have large showy flowers, others more I. ‘Black Gamecock’ understated; some grow in clumps, others spread; some prefer I. ensata ‘Angelic Choir’ it dry, others are more partial to moist, even wet conditions; and some grow from bulbs, while others return each year from rhizomes just beneath the soil surface. How does one tell them apart and make the right choice for a home garden? Fortunately, horticulturists and iris enthusiasts have developed a system of organization to make sense out of the vast world of irises. Three groups that account for more than 75% of the commercial iris market today are the Bearded Iris, Siberian Iris, and Japanese Iris. Each group recognizes the best of the best with prestigious national awards, noted in the descriptions that follow. The Dykes Medal is awarded to the finest iris of any class. More iris plants are described in the “Plant Descriptions: I. ×pseudata ‘Aichi no Kagayaki’ I. ensata ‘Cascade Crest’ Perennial” section. Latin Name Common Name Mature Size Light Soil Pot Size Price Iris ‘Black Gamecock’ Louisiana Iris 2–3 .8 d 1 g $14 Late; stunning blue black, velvet-colored flowers; hummingbird haven; can grow in 4 inches of standing water; DeBaillon Medal. Iris ×pseudata ‘Aichi no Kagayaki’ Iris Hybrid 2 . -

The ISCC-NBS Method of Designating Colors and a Dictionary of Color Names
Uc 8 , .Department of Commerce Na Canal Bureau of Standards Circular UNITED STATES DEPARTMENT OF COMMERCE • Sinclair Weeks, Secretary NATIONAL BUREAU OF STANDARDS • A. V. Astin, Director The ISCC-NBS Method of Designating Colors and a Dictionary of Color Names National Bureau of Standards Circular 553 Issued November 1, 1955 For sale by the Superintendent of Documents, U. S. Government Printing Office, Washington 25, D. C. Price 32 7 1 National Bureau of Standards NOV 1 1955 8 (0*118 QC 00 U555 Cop. 1 Preface I^Ever since the language of man began to develop, words or expressions have been used first to indicate and then to describe colors. Some of these have per- sisted throughout the centuries and are those which refer to the simple colors or ranges such as red or yellow. As the language developed, more and more color names were invented to describe the colors used by art and industry and in late years in the rapidly expanding field of sales promotion. Some of these refer to the pigment or dye used, as Ochre Red or Cochineal, or a geographical location of its source such as Naples Yellow or Byzantium. Later when it became clear that most colors are bought by or for women, many color names indicative of the beauties and wiles of the fan- sex were introduced, as French Nude, Heart’s Desire, Intimate Mood, or Vamp. Fanciful color names came into vogue such as Dream Fluff, Happy Day, Pearly Gates, and Wafted Feather. Do not suppose that these names are without economic importance for a dark reddish gray hat for Milady might be a best seller ; if advertised as Mauve Wine whereas it probably would not if the color were called Paris Mud. -

CREATOR 100 Per Box
UNISEX T-SHIRT STTU755 - CREATOR 100 per box Iconic Unisex T-Shirt Singlejersey 100% gekämmte ringgesponnene Bio-Baumwolle Normale Passform 180 GSM . Eingesetzte Ärmel . 1x1 Rippstrick am Halsausschnitt . Abgesetztes Nackenband . Breite Doppelabsteppung an Ärmelenden und unterem Saum C WHITES 2.5cm below A armhole B WHITE OFF WHITE VINTAGE WHITE NATURAL RAW COLORS FRONT BACK SIZES XXS XS S M L XL XXL XXXL XXXXL XXXXXL BLACK ANTHRACITE OPAL DESERT DUST CAMEL DEEP CHOCOLATE CARAMEL MUSHROOM OCHRE A Half Chest 43.5 46 49 52 55 58 61 64 69 74 B Body Length 64 66 69 72 74 76 78 80 82 84 C Sleeve Length 19 19.5 20.5 21.5 22.5 22.5 23.5 24.5 24.5 25 Some sizes are only available on a limited number of colours. Please check our webshop for latest information SPECTRA YELLOW GOLDEN YELLOW FRESH GREEN VARSITY GREEN GLAZED GREEN BOTTLE GREEN BRITISH KHAKI KHAKI SAGE Combo options STEM GREEN CARIBBEAN BLUE TEAL MONSTERA OCEAN DEPTH STARGAZER CITADEL BLUE BABY BLUE SERENE BLUE SKY BLUE BRIGHT BLUE AZUR ROYAL BLUE MAJORELLE BLUE INDIA INK GREY FRENCH NAVY PINK PUNCH CARMINE RED RED Mini Creator Stella Expresser BURGUNDY PLUM LAVENDER DAWN LILAC PETAL MAUVE CANDY PINK COTTON PINK ROSE CLAY MELON CODE Printing Specifications NEWNEW NEWNEW NEWNEW NEWNEW NEWNEW NEWNEW Eignet sich für alle Drucktechniken. Mehr Informationen finden Sie auf unserer Website. BRIGHT ORANGE TANGERINE BRIGHT RED JOJOBA INDIGO HUSH LAVENDER ORCHID FLOWER DUSTY MINT HIBISCUS ROSE Certifications & memberships ESSENTIAL HEATHERS DARK HEATHER MID HEATHER GREY HEATHER GREY CREAM HEATHER HEATHER SAND MID HEATHER MID HEATHER HEATHER ICE BLUE MID HEATHER BLUE GREY GREY KHAKI GREEN Wash Instructions Mit ähnlichen Farben waschen, Aufdruck nicht bügeln, auf links waschen und bügeln. -

Development of a Genetic Multicolor Cell Labeling Approach for Neural
Development of a genetic multicolor cell labeling approach for neural circuit analysis in Drosophila Dafni Hadjieconomou January 2013 Division of Molecular Neurobiology MRC National Institute for Medical Research The Ridgeway Mill Hill, London NW7 1AA U.K. Department of Cell and Developmental Biology University College London A thesis submitted to the University College London for the degree of Doctor of Philosophy Declaration of authenticity This work has been completed in the laboratory of Iris Salecker, in the Division of Molecular Neurobiology at the MRC National Institute for Medical Research. I, Dafni Hadjieconomou, declare that the work presented in this thesis is the result of my own independent work. Any collaborative work or data provided by others have been indicated at respective chapters. Chapters 3 and 5 include data generated and kindly provided by Shay Rotkopf and Iris Salecker as indicated. 2 Acknowledgements I would like to express my utmost gratitude to my supervisor, Iris Salecker, for her valuable guidance and support throughout the entire course of this PhD. Working with you taught me to work with determination and channel my enthusiasm in a productive manner. Thank you for sharing your passion for science and for introducing me to the colourful world of Drosophilists. Finally, I must particularly express my appreciation for you being very understanding when times were difficult, and for your trust in my successful achieving. Many thanks to my thesis committee, Alex Gould, James Briscoe and Vassilis Pachnis for their quidance during this the course of this PhD. I am greatly thankful to all my colleagues and friends in the lab. -

Pennsylvania Folklife Vol. 36, No. 3 Michael Colby
Ursinus College Digital Commons @ Ursinus College Pennsylvania Folklife Magazine Pennsylvania Folklife Society Collection Spring 1987 Pennsylvania Folklife Vol. 36, No. 3 Michael Colby Donald Graves Monica Pieper William T. Parsons Ursinus College Helen Urda Smith Follow this and additional works at: https://digitalcommons.ursinus.edu/pafolklifemag Part of the American Art and Architecture Commons, American Material Culture Commons, Christian Denominations and Sects Commons, Cultural History Commons, Ethnic Studies Commons, Fiber, Textile, and Weaving Arts Commons, Folklore Commons, Genealogy Commons, German Language and Literature Commons, Historic Preservation and Conservation Commons, History of Religion Commons, Linguistics Commons, and the Social and Cultural Anthropology Commons Click here to let us know how access to this document benefits oy u. Recommended Citation Colby, Michael; Graves, Donald; Pieper, Monica; Parsons, William T.; and Smith, Helen Urda, "Pennsylvania Folklife Vol. 36, No. 3" (1987). Pennsylvania Folklife Magazine. 116. https://digitalcommons.ursinus.edu/pafolklifemag/116 This Book is brought to you for free and open access by the Pennsylvania Folklife Society Collection at Digital Commons @ Ursinus College. It has been accepted for inclusion in Pennsylvania Folklife Magazine by an authorized administrator of Digital Commons @ Ursinus College. For more information, please contact [email protected]. I------.w'l_____ -----,.-~ ~tnn~ lJ {vania oeoeoeoeoeoeoeoeoeoeo ul Ii e (tontril1utor~ MI C HAEL COLBY, a teacher in the Bethlehem A rea School District, a nd DO ALD G RAVES, a freela nce wri ter for the Bethlehem Globe Times a nd Early American Life magazine, are deeply in volved in 18th century life a nd traditions. T hey have bee n growing a nd ha nd -processing fl ax- spinning, dyeing a nd weaving the fi ber into cloth as was done by the settlers in colonial Pennsylva nia- a nd have been connected with the Jacobsburg Environmental Center a nd Historic Bethlehem, Inc. -

CREATOR 100 Per Box
UNISEX T-SHIRTS CREATOR 100 per box STTU755 The iconic unisex t-shirt Single Jersey 100% Organic ring-spun combed cotton 180 GSM . Set-in sleeve . 1x1 rib at neck collar . Inside back neck tape in self fabric . Sleeve hem and bottom hem with wide double needle topstitch MEDIUM FIT WHITES XXS* XS S M L XL XXL XXXL* XXXXL* XXXXXL* NEWNEW Size guide NATURAL RAW VINTAGE WHITE OFF WHITE WHITE COLORS C NEWNEW NEWNEW NEWNEW NEWNEW NEWNEW NEWNEW NEWNEW NEWNEW 2.5cm below A armhole CARAMEL BRIGHT BLUE MELON CODE CARMINE RED SAGE ROSE CLAY OCHRE MAUVE BRIGHT RED NEWNEW B FRONT BACK TANGERINE BRIGHT ORANGE SUNSET ORANGE CANDY PINK BURGUNDY RED PINK PUNCH COTTON PINK LAVENDER DAWN SIZES XXS XS S M L XL XXL XXXL XXXXL XXXXXL A Half Chest 43.5 46 49 52 55 58 61 64 69 74 PLUM MAJORELLE BLUE ROYAL BLUE FRENCH NAVY AZUR SKY BLUE BABY BLUE CITADEL BLUE STARGAZER B Body Length 64 66 69 72 74 76 78 80 82 84 C Sleeve Length 19 19.5 20.5 21.5 22.5 22.5 23.5 24.5 24.5 25 OCEAN DEPTH CARIBBEAN BLUE FRESH GREEN VARSITY GREEN GOLDEN YELLOW SPECTRA YELLOW HAY YELLOW MOSS GREEN BRITISH KHAKI Combo options KHAKI GLAZED GREEN BOTTLE GREEN DEEP CHOCOLATE ROASTED ORANGE CAMEL DESERT DUST OPAL ANTHRACITE INDIA INK GREY BLACK Stella Expresser Mini Creator ESSENTIAL HEATHERS Printing Specifications Fits all kinds of print techniques. Please download our printing guide for CREAM HEATHER MID HEATHER RED DARK HEATHER DARK HEATHER MID HEATHER BLUE HEATHER ICE BLUE MID HEATHER MID HEATHER HEATHER SAND PINK INDIGO BLUE GREEN KHAKI details. -

Towards a Standard Terminology for Describing Academic Dress
Transactions of the Burgon Society Volume 1 Article 2 1-1-2001 Towards a Standard Terminology for Describing Academic Dress Nicholas Groves Follow this and additional works at: https://newprairiepress.org/burgonsociety Recommended Citation Groves, Nicholas (2001) "Towards a Standard Terminology for Describing Academic Dress," Transactions of the Burgon Society: Vol. 1. https://doi.org/10.4148/2475-7799.1001 This Article is brought to you for free and open access by New Prairie Press. It has been accepted for inclusion in Transactions of the Burgon Society by an authorized administrator of New Prairie Press. For more information, please contact [email protected]. BBurgon Society Annual, 2001, pp. 9–12 TOWARDS A STANDARD TERMINOLOGY FOR DESCRIBING ACADEMIC ROBES Nicholas Groves, MA, BMus, FBS, FSAScot It has been clear for many years that a standard, clear, terminology for describing academic robes is needed. Universities and colleges use very imprecise terms, and different institutions will use the same term with different meanings. A standard terminology should enable a gown or hood to be drawn accurately from its description, exactly as an heraldic blazon enables a coat-of-arms to be drawn. 1. Patterns/shapes. A start has already been made here with my classificatory system, whereby the different patterns of full, simple and Aberdeen hoods are each assigned a number, and the various shapes of robes and gowns are similarly codified (see Appendix I). There are probably a few more to be added, and some apparently differing patterns are assigned the same number – e.g. the ‘Leeds’ version of the full hood (with short cowl) is assigned the [f1] of Cambridge, as the length of the cowl is of no importance; likewise ‘London’ pattern doctors robes are listed as Cambridge [d1] as the London version is a very recent deviation. -

Made in America
Made in America www.bobbincentral.com Color SKU 1000m 5000m White 8 10000 Super White 10002 German Granite 8 10401 Medium Grey 8 10424 Battleship 10430 Titanium 8 10431 Flint 10435 Silver 10536 Mercury 10643 Sterling 10877 Cool Grey 3 8 10CG3 Fog 10CG6 Cool Grey 7 8 10CG7 Cool Grey 9 10CG9 Linen 8 10WG1 Warm Grey 4 8 10WG4 Warm Grey 6 10WG6 Black 8 11001 Slate 15285 Anchor 15295 Nickel 15497 Bone 17443 Light Grey 8 17543 Shadow 1BLK3 Storm 1BLK7 Lead Grey 8 1CG11 Warm Grey 11 1WG11 Cream 8 20001 Pearl 20005 Leather 8 20140 Mahogany 8 20160 Medium Brown 8 20464 Sand 8 20466 Caramel 20467 Biscotti 20468 Chocolate 20469 Hazel 20470 Apricot Blush 20474 Dark Brown 8 20476 Rust Brown 8 20478 MoCha 8 20727 Light Copper 20730 Vegas Gold 20872 Dijon 21245 Antique 21255 Sienna 21615 Sepia 21685 Cleopatra 8 24515 Khaki 8 24525 Shell 24535 Brunette 8 24625 Bark 24635 Light Tan 8 24655 Camel 24665 Cork 24675 Cocoa 24705 Military Gold 8 27407 Wheat 27500 Sand Dune 27501 Coffee 27504 Butterscotch 27508 Coffee Bean 27518 Chestnut 8 27521 Auburn 27523 Brownie 27596 Latte 29181 Rock Navy 30001 Blueberry 8 30281 Azure 30283 Hawaiian Blue 8 30284 Empire 30286 Bombay 30287 Bright Blue 8 30288 Baby Blue 30290 Midnight Navy 30296 Cerulean 30308 Magic Mint 30317 Eclipse 30532 Denim 30534 Robin Egg 8 30632 Graphite 30644 Sky 30646 Cobalt 30647 Admiral 30654 Captain Navy 30655 Royal 30661 Lagoon 32237 Air ForCe Blue 32382 Federal 32757 Deep Sea 32767 Navy 8 32965 Light Turquoise 8 32975 Electric 33015 Zaffre 35405 Cloud 8 37457 Ocean 37468 Aquamarine 37474 Steel -

Northern Spain & Portugal: Pilgrimage Into the Past 2023
YOUR O.A.T. ADVENTURE TRAVEL PLANNING GUIDE® Northern Spain & Portugal: Pilgrimage into the Past 2023 Small Groups: 8-16 travelers—guaranteed! (average of 13) Overseas Adventure Travel ® The Leader in Personalized Small Group Adventures on the Road Less Traveled 1 Dear Traveler, For me, one of the joys of traveling is the careful planning that goes into an adventure—from the first spark of inspiration to hours spent poring over travel books about my dream destinations—and I can’t wait to see where my next journey will take me. I know you’re eager to explore the world, too, and our Northern Spain & Portugal itinerary described inside is an excellent way to start. Exactly how your adventure unfolds is up to you, because you have many choices to customize it. You can arrive early and stay later—perhaps by adding a pre- or post-trip extension, spending time in a Stopover city, or combining 2 or more trips. Plus, your itinerary is laced with free time, so you’ll have opportunities to do your own thing. More than 80% of the travelers who reserve this trip choose to tailor their adventure. In fact, O.A.T. is the only travel company to offer such flexibility and choice for an experience that is truly personalized. As for Northern Spain & Portugal, thanks to your small group of 8-16 travelers (average 13) you can expect some unforgettable experiences. Here are a few that stood out for me: When I stepped into Santa Colomba de Somoza, a rural village nestled in Spain’s northern León province, I felt an overwhelming sense of community—a small group bonded by their unique cultural identity as Maragatos, believed to be the last living descendents of the Berbers. -

—Hence, Tirade
e. —hence , t irad to: literary a misfits digital monthly from: robocup zine press FEBRUARY 2018 | THE LOVE + HATE ISSUE. I don't know how to write love letters. -Frida Kahlo t w o table of contents plasmatic valentine 2 by tamryn spruill micro poem from The Twitter Hive Mind Is Dreaming 5 by jackie wang lost color 6 by katy ilonka i do 7 by judy swann love & hate playlists 8 by nottheboyevan monthly [horoscapes] 10 by matthew burnside blue is the warmest color movie review 12 by her head in films knock knock 13 by j. bradley coming next month ... 14 tuesday 15 by adrian slonaker nothing more to tell 16 by mitchell krockmalnik grabois the collection 20 by sarah etlinger contributor bios 22 acknowledgments 25 —hence, tirade. f SUBMIT o SUBSCRIBE u r www.robocup-press.com Copyright 2018 © Robocup Press. I'm beingI'm being cooked from thecooked from the inside by myinside by my desire. desire. -Jackie Wang Pre-orders for her forthcoming Twitter-poem collection begin 3/3! 5 Lost Color Katy Ilonka I cannot find it. Pushed bark into bloodshot birds and the irises that smell of the times you were here and it has gone. I have heard of a professor who studies the passage of time building a machine that brings the eyes into a single space and places the color precisely. I have heard of a yogi mechanic who adjusts the color with a chakra wrench until it glows just right. I have heard of the times our steps were in sync. -

European Cobalt Sources Identified in the Production of Chinese Famille Rose Porcelain Abstract Keywords
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery 1 European cobalt sources identified in the production of 2 Chinese famille rose porcelain 3 4 Rita Giannini1, Ian C. Freestone2, Andrew J. Shortland1 5 6 1 Cranfield Forensic Institute 7 Cranfield University 8 Defence Academy of the United Kingdom 9 Shrivenham 10 Wilts 11 SN6 8LA 12 [email protected] 13 14 2 Institute of Archaeology 15 UCL 16 31-34 Gordon Square 17 London WC1H 0PY 18 Abstract 19 The blue pigments on 112 fragments or small objects of Qing Dynasty Chinese, 20 95 of underglaze blue and white and 17 overglaze enamelled porcelains were 21 analysed by LA-ICPMS. The underglaze blues on both blue and white and 22 polychrome objects were created with a cobalt pigment that was rich in 23 manganese with lesser nickel and zinc. This suite of accessory elements is 24 generally considered to be characteristic of local, Chinese, sources of pigments. 25 However, the blue enamels were very different. The cobalt pigment here has low 26 levels of manganese and instead is rich in nickel, zinc, arsenic and bismuth. No 27 Chinese source of cobalt with these characteristics is known, but they closely 28 match the elements found in the contemporary cobalt source at Erzgebirge in 29 Germany. Textual evidence has been interpreted to suggest that some enamel 30 pigment technologies were transferred from Europe to China, but this is the first 31 analytical evidence to be found that an enamel pigment itself was imported. -
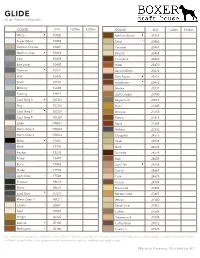
Thread Color Chart BCH.Pdf
GLIDE 40 wt. Filament Polyester Order a color card for a true color match Magna-Glide Delights available in this color COLOR SKU 1,000m 5,000m COLOR SKU 1,000m 5,000m White 10000 Medium Brown 20464 Super White 10002 Sand 20466 German Granite 10401 Caramel 20467 Medium Grey 10424 Biscotti 20468 Coin 10429 Chocolate 20469 Battleship 10430 Hazel 20470 Titanium 10431 Apricot Blush 20474 Flint 10435 Dark Brown 20476 Silver 10536 Rust Brown 20478 Mercury 10643 Mocha 20727 Sterling 10877 Light Copper 20730 Cool Grey 3 10CG3 Vegas Gold 20872 Fog 10CG6 Dijon 21245 Cool Grey 7 10CG7 Antique 21255 Cool Grey 9 10CG9 Sienna 21615 Linen 10WG1 Sepia 21685 Warm Grey 4 10WG4 Hickory 22336 Warm Grey 6 10WG6 Cleopatra 24515 Black 11001 Khaki 24525 Slate 15285 Shell 24535 Anchor 15295 Brunette 24625 Nickel 15497 Bark 24635 Bone 17443 Light Tan 24655 Husky 17530 Camel 24665 Light Grey 17543 Cork 24675 Shadow 1BLK3 Cocoa 24705 Storm 1BLK7 Buttermilk 27403 Lead Grey 1CG11 Military Gold 27407 Warm Grey 11 1WG11 Wheat 27500 Cream 20001 Sand Dune 27501 Pearl 20005 Coffee 27504 Ginger 20125 Butterscotch 27508 Leather 20140 Coffee Bean 27518 Mahogany 20160 Chestnut 27521 Our color chart is designed to help you reference Glide™ thread colors. We offer this chart as a reference only, and it is not intended to imply an exact color match of Glide™ thread colors. Color appearance will vary based on lighting conditions and angle of view. www.habanddash.com Made in America, Stitched by All GLIDE 40 wt. Filament Polyester Order a color card for a true color match Magna-Glide