Molecular Detection of Rickettsia Spp. and Coxiella Burnetii in Cattle, Water Bu Ffalo, and Rhipicephalus (Boophilus) Microplus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Parinaud's Oculoglandular Syndrome
Tropical Medicine and Infectious Disease Case Report Parinaud’s Oculoglandular Syndrome: A Case in an Adult with Flea-Borne Typhus and a Review M. Kevin Dixon 1, Christopher L. Dayton 2 and Gregory M. Anstead 3,4,* 1 Baylor Scott & White Clinic, 800 Scott & White Drive, College Station, TX 77845, USA; [email protected] 2 Division of Critical Care, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA; [email protected] 3 Medical Service, South Texas Veterans Health Care System, San Antonio, TX 78229, USA 4 Division of Infectious Diseases, Department of Medicine, University of Texas Health, San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229, USA * Correspondence: [email protected]; Tel.: +1-210-567-4666; Fax: +1-210-567-4670 Received: 7 June 2020; Accepted: 24 July 2020; Published: 29 July 2020 Abstract: Parinaud’s oculoglandular syndrome (POGS) is defined as unilateral granulomatous conjunctivitis and facial lymphadenopathy. The aims of the current study are to describe a case of POGS with uveitis due to flea-borne typhus (FBT) and to present a diagnostic and therapeutic approach to POGS. The patient, a 38-year old man, presented with persistent unilateral eye pain, fever, rash, preauricular and submandibular lymphadenopathy, and laboratory findings of FBT: hyponatremia, elevated transaminase and lactate dehydrogenase levels, thrombocytopenia, and hypoalbuminemia. His condition rapidly improved after starting doxycycline. Soon after hospitalization, he was diagnosed with uveitis, which responded to topical prednisolone. To derive a diagnostic and empiric therapeutic approach to POGS, we reviewed the cases of POGS from its various causes since 1976 to discern epidemiologic clues and determine successful diagnostic techniques and therapies; we found multiple cases due to cat scratch disease (CSD; due to Bartonella henselae) (twelve), tularemia (ten), sporotrichosis (three), Rickettsia conorii (three), R. -
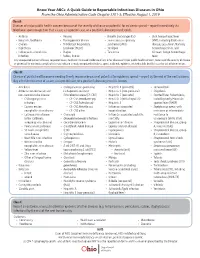
Know Your Abcs: a Quick Guide to Reportable Infectious Diseases in Ohio
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective August 1, 2019 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Measles • Rubella (not congenital) • Viral hemorrhagic fever • Botulism, foodborne • Meningococcal disease • Severe acute respiratory (VHF), including Ebola virus • Cholera • Middle East Respiratory syndrome (SARS) disease, Lassa fever, Marburg • Diphtheria Syndrome (MERS) • Smallpox hemorrhagic fever, and • Influenza A – novel virus • Plague • Tularemia Crimean-Congo hemorrhagic infection • Rabies, human fever Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis B (perinatal) • Salmonellosis • Arboviral neuroinvasive and carbapenem-resistant • Hepatitis C (non-perinatal) • Shigellosis -

Genome Project Reveals a Putative Rickettsial Endosymbiont
GBE Bacterial DNA Sifted from the Trichoplax adhaerens (Animalia: Placozoa) Genome Project Reveals a Putative Rickettsial Endosymbiont Timothy Driscoll1,y, Joseph J. Gillespie1,2,*,y, Eric K. Nordberg1,AbduF.Azad2, and Bruno W. Sobral1,3 1Virginia Bioinformatics Institute at Virginia Polytechnic Institute and State University 2Department of Microbiology and Immunology, University of Maryland School of Medicine 3Present address: Nestle´ Institute of Health Sciences SA, Campus EPFL, Quartier de L’innovation, Lausanne, Switzerland *Corresponding author: E-mail: [email protected]. yThese authors contributed equally to this work. Accepted: March 1, 2013 Abstract Eukaryotic genome sequencing projects often yield bacterial DNA sequences, data typically considered as microbial contamination. However, these sequences may also indicate either symbiont genes or lateral gene transfer (LGT) to host genomes. These bacterial sequences can provide clues about eukaryote–microbe interactions. Here, we used the genome of the primitive animal Trichoplax adhaerens (Metazoa: Placozoa), which is known to harbor an uncharacterized Gram-negative endosymbiont, to search for the presence of bacterial DNA sequences. Bioinformatic and phylogenomic analyses of extracted data from the genome assembly (181 bacterial coding sequences [CDS]) and trace read archive (16S rDNA) revealed a dominant proteobacterial profile strongly skewed to Rickettsiales (Alphaproteobacteria) genomes. By way of phylogenetic analysis of 16S rDNA and 113 proteins conserved across proteobacterial genomes, as well as identification of 27 rickettsial signature genes, we propose a Rickettsiales endosymbiont of T. adhaerens (RETA). The majority (93%) of the identified bacterial CDS belongs to small scaffolds containing prokaryotic-like genes; however, 12 CDS were identified on large scaffolds comprised of eukaryotic-like genes, suggesting that T. -

Melioidosis in Northern Tanzania: an Important Cause of Febrile Illness?
Melioidosis and serological evidence of exposure to Burkholderia pseudomallei among patients with fever, northern Tanzania Michael Maze Department of Medicine University of Otago, Christchurch Melioidosis Melioidosis caused by Burkholderia pseudomallei • Challenging to identify when cultured Soil reservoir, with human infection from contact with contaminated water Most infected people are asymptomatic: 1 clinical illness for 4,500 antibody producing exposures Febrile illness with a variety of presentations and bacteraemia is present in 40-60% of people with acute illness Estimated 89,000 deaths globally Gavin Koh CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=4975784 Laboratory diagnosis of febrile inpatients, northern Tanzania, 2007-8 (n=870) Malaria (1.6%) Bacteremia (9.8%) Mycobacteremia (1.6%) Fungemia (2.9%) Brucellosis (3.5%) Leptospirosis (8.8%) Q fever (5.0%) No diagnosis (50.1%) Spotted fever group rickettsiosis (8.0%) Typhus group rickettsiosis (0.4%) Chikungunya (7.9%) Crump PLoS Neglect Trop Dis 2013; 7: e2324 Predicted environmental suitability for B. pseudomallei in East Africa Large areas of Africa are predicted to be highly suitable for Burkholderia pseudomallei East Africa is less suitable but pockets of higher suitability including northern Tanzania Northern Tanzania Increasing suitability for B. pseudomallei Limmathurotsakul Nat Microbiol. 2016;1(1). Melioidosis epidemiology in Africa Paucity of empiric data • Scattered case reports • Report from Kilifi, Kenya identified 4 bacteraemic cases from 66,000 patients who had blood cultured1 • 5.9% seroprevalence among healthy adults in Uganda2 • No reports from Tanzania 1. Limmathurotsakul Nat Microbiol. 2016;1(1). 2. Frazer J R Army Med Corps. 1982;128(3):123-30. -

Ohio Department of Health, Bureau of Infectious Diseases Disease Name Class A, Requires Immediate Phone Call to Local Health
Ohio Department of Health, Bureau of Infectious Diseases Reporting specifics for select diseases reportable by ELR Class A, requires immediate phone Susceptibilities specimen type Reportable test name (can change if Disease Name other specifics+ call to local health required* specifics~ state/federal case definition or department reporting requirements change) Culture independent diagnostic tests' (CIDT), like BioFire panel or BD MAX, E. histolytica Stain specimen = stool, bile results should be sent as E. histolytica DNA fluid, duodenal fluid, 260373001^DETECTED^SCT with E. histolytica Antigen Amebiasis (Entamoeba histolytica) No No tissue large intestine, disease/organism-specific DNA LOINC E. histolytica Antibody tissue small intestine codes OR a generic CIDT-LOINC code E. histolytica IgM with organism-specific DNA SNOMED E. histolytica IgG codes E. histolytica Total Antibody Ova and Parasite Anthrax Antibody Anthrax Antigen Anthrax EITB Acute Anthrax EITB Convalescent Anthrax Yes No Culture ELISA PCR Stain/microscopy Stain/spore ID Eastern Equine Encephalitis virus Antibody Eastern Equine Encephalitis virus IgG Antibody Eastern Equine Encephalitis virus IgM Arboviral neuroinvasive and non- Eastern Equine Encephalitis virus RNA neuroinvasive disease: Eastern equine California serogroup virus Antibody encephalitis virus disease; LaCrosse Equivocal results are accepted for all California serogroup virus IgG Antibody virus disease (other California arborviral diseases; California serogroup virus IgM Antibody specimen = blood, serum, serogroup -

Mechanisms of Rickettsia Parkeri Invasion of Host Cells and Early Actin-Based Motility
Mechanisms of Rickettsia parkeri invasion of host cells and early actin-based motility By Shawna Reed A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Microbiology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Matthew D. Welch, Chair Professor David G. Drubin Professor Daniel Portnoy Professor Kathleen R. Ryan Fall 2012 Mechanisms of Rickettsia parkeri invasion of host cells and early actin-based motility © 2012 By Shawna Reed ABSTRACT Mechanisms of Rickettsia parkeri invasion of host cells and early actin-based motility by Shawna Reed Doctor of Philosophy in Microbiology University of California, Berkeley Professor Matthew D. Welch, Chair Rickettsiae are obligate intracellular pathogens that are transmitted to humans by arthropod vectors and cause diseases such as spotted fever and typhus. Spotted fever group (SFG) Rickettsia hijack the host actin cytoskeleton to invade, move within, and spread between eukaryotic host cells during their obligate intracellular life cycle. Rickettsia express two bacterial proteins that can activate actin polymerization: RickA activates the host actin-nucleating Arp2/3 complex while Sca2 directly nucleates actin filaments. In this thesis, I aimed to resolve which host proteins were required for invasion and intracellular motility, and to determine how the bacterial proteins RickA and Sca2 contribute to these processes. Although rickettsiae require the host cell actin cytoskeleton for invasion, the cytoskeletal proteins that mediate this process have not been completely described. To identify the host factors important during cell invasion by Rickettsia parkeri, a member of the SFG, I performed an RNAi screen targeting 105 proteins in Drosophila melanogaster S2R+ cells. -

Gene Gain and Loss Events in Rickettsia and Orientia Species Kalliopi Georgiades1,2, Vicky Merhej1, Khalid El Karkouri1, Didier Raoult1, Pierre Pontarotti2*
Georgiades et al. Biology Direct 2011, 6:6 http://www.biology-direct.com/content/6/1/6 RESEARCH Open Access Gene gain and loss events in Rickettsia and Orientia species Kalliopi Georgiades1,2, Vicky Merhej1, Khalid El Karkouri1, Didier Raoult1, Pierre Pontarotti2* Abstract Background: Genome degradation is an ongoing process in all members of the Rickettsiales order, which makes these bacterial species an excellent model for studying reductive evolution through interspecies variation in genome size and gene content. In this study, we evaluated the degree to which gene loss shaped the content of some Rickettsiales genomes. We shed light on the role played by horizontal gene transfers in the genome evolution of Rickettsiales. Results: Our phylogenomic tree, based on whole-genome content, presented a topology distinct from that of the whole core gene concatenated phylogenetic tree, suggesting that the gene repertoires involved have different evolutionary histories. Indeed, we present evidence for 3 possible horizontal gene transfer events from various organisms to Orientia and 6 to Rickettsia spp., while we also identified 3 possible horizontal gene transfer events from Rickettsia and Orientia to other bacteria. We found 17 putative genes in Rickettsia spp. that are probably the result of de novo gene creation; 2 of these genes appear to be functional. On the basis of these results, we were able to reconstruct the gene repertoires of “proto-Rickettsiales” and “proto-Rickettsiaceae”, which correspond to the ancestors of Rickettsiales and Rickettsiaceae, respectively. Finally, we found that 2,135 genes were lost during the evolution of the Rickettsiaceae to an intracellular lifestyle. Conclusions: Our phylogenetic analysis allowed us to track the gene gain and loss events occurring in bacterial genomes during their evolution from a free-living to an intracellular lifestyle. -

Scrub Typhus and Molecular Characterization of Orientia Tsutsugamushi from Central Nepal
pathogens Article Scrub Typhus and Molecular Characterization of Orientia tsutsugamushi from Central Nepal Rajendra Gautam 1, Keshab Parajuli 1, Mythili Tadepalli 2, Stephen Graves 2, John Stenos 2,* and Jeevan Bahadur Sherchand 1 1 Department of Microbiology, Maharajgunj Medical Campus, Institute of Medicine, Kathmandu 44600, Nepal; [email protected] (R.G.); [email protected] (K.P.); [email protected] (J.B.S.) 2 Australian Rickettsial Reference Laboratory, Geelong, VIC 3220, Australia; [email protected] (M.T.); [email protected] (S.G.) * Correspondence: [email protected]; Tel.: +61-342151357 Abstract: Scrub typhus is a vector-borne, acute febrile illness caused by Orientia tsutsugamushi. Scrub typhus continues to be an important but neglected tropical disease in Nepal. Information on this pathogen in Nepal is limited to serological surveys with little information available on molecular methods to detect O. tsutsugamushi. Limited information exists on the genetic diversity of this pathogen. A total of 282 blood samples were obtained from patients with suspected scrub typhus from central Nepal and 84 (30%) were positive for O. tsutsugamushi by 16S rRNA qPCR. Positive samples were further subjected to 56 kDa and 47 kDa molecular typing and molecularly compared to other O. tsutsugamushi strains. Phylogenetic analysis revealed that Nepalese O. tsutsugamushi strains largely cluster together and cluster away from other O. tsutsugamushi strains from Asia and elsewhere. One exception was the sample of Nepal_1, with its partial 56 kDa sequence clustering Citation: Gautam, R.; Parajuli, K.; more closely with non-Nepalese O. tsutsugamushi 56 kDa sequences, potentially indicating that Tadepalli, M.; Graves, S.; Stenos, J.; homologous recombination may influence the genetic diversity of strains in this region. -

Typhus Fever, Organism Inapparently
Rickettsia Importance Rickettsia prowazekii is a prokaryotic organism that is primarily maintained in prowazekii human populations, and spreads between people via human body lice. Infected people develop an acute, mild to severe illness that is sometimes complicated by neurological Infections signs, shock, gangrene of the fingers and toes, and other serious signs. Approximately 10-30% of untreated clinical cases are fatal, with even higher mortality rates in Epidemic typhus, debilitated populations and the elderly. People who recover can continue to harbor the Typhus fever, organism inapparently. It may re-emerge years later and cause a similar, though Louse–borne typhus fever, generally milder, illness called Brill-Zinsser disease. At one time, R. prowazekii Typhus exanthematicus, regularly caused extensive outbreaks, killing thousands or even millions of people. This gave rise to the most common name for the disease, epidemic typhus. Epidemic typhus Classical typhus fever, no longer occurs in developed countries, except as a sporadic illness in people who Sylvatic typhus, have acquired it while traveling, or who have carried the organism for years without European typhus, clinical signs. In North America, R. prowazekii is also maintained in southern flying Brill–Zinsser disease, Jail fever squirrels (Glaucomys volans), resulting in sporadic zoonotic cases. However, serious outbreaks still occur in some resource-poor countries, especially where people are in close contact under conditions of poor hygiene. Epidemics have the potential to emerge anywhere social conditions disintegrate and human body lice spread unchecked. Last Updated: February 2017 Etiology Rickettsia prowazekii is a pleomorphic, obligate intracellular, Gram negative coccobacillus in the family Rickettsiaceae and order Rickettsiales of the α- Proteobacteria. -

Tick-Borne Disease Working Group 2020 Report to Congress
2nd Report Supported by the U.S. Department of Health and Human Services • Office of the Assistant Secretary for Health Tick-Borne Disease Working Group 2020 Report to Congress Information and opinions in this report do not necessarily reflect the opinions of each member of the Working Group, the U.S. Department of Health and Human Services, or any other component of the Federal government. Table of Contents Executive Summary . .1 Chapter 4: Clinical Manifestations, Appendices . 114 Diagnosis, and Diagnostics . 28 Chapter 1: Background . 4 Appendix A. Tick-Borne Disease Congressional Action ................. 8 Chapter 5: Causes, Pathogenesis, Working Group .....................114 and Pathophysiology . 44 The Tick-Borne Disease Working Group . 8 Appendix B. Tick-Borne Disease Working Chapter 6: Treatment . 51 Group Subcommittees ...............117 Second Report: Focus and Structure . 8 Chapter 7: Clinician and Public Appendix C. Acronyms and Abbreviations 126 Chapter 2: Methods of the Education, Patient Access Working Group . .10 to Care . 59 Appendix D. 21st Century Cures Act ...128 Topic Development Briefs ............ 10 Chapter 8: Epidemiology and Appendix E. Working Group Charter. .131 Surveillance . 84 Subcommittees ..................... 10 Chapter 9: Federal Inventory . 93 Appendix F. Federal Inventory Survey . 136 Federal Inventory ....................11 Chapter 10: Public Input . 98 Appendix G. References .............149 Minority Responses ................. 13 Chapter 11: Looking Forward . .103 Chapter 3: Tick Biology, Conclusion . 112 Ecology, and Control . .14 Contributions U.S. Department of Health and Human Services James J. Berger, MS, MT(ASCP), SBB B. Kaye Hayes, MPA Working Group Members David Hughes Walker, MD (Co-Chair) Adalbeto Pérez de León, DVM, MS, PhD Leigh Ann Soltysiak, MS (Co-Chair) Kevin R. -

Intraspecies Comparative Genomics of Rickettsia
AIX ͲMARSEILLE UNIVERSITÉ FACULTÉ DE MÉDECINE DE MARSEILLE ÉCOLE DOCTORALE DES SCIENCES DE LA VIE ET DE LA SANTÉ T H È S E Présentée et publiquement soutenue devant LA FACULTÉ DE MÉDECINE DE MARSEILLE Le 13 décembre 2013 Par M. Erwin SENTAUSA Né le 16 décembre 1979 àMalang, Indonésie INTRASPECIES COMPARATIVE GENOMICS OF RICKETTSIA Pour obtenir le grade de DOCTORAT d’AIX ͲMARSEILLE UNIVERSITÉ SPÉCIALITÉ :PATHOLOGIE HUMAINE Ͳ MALADIES INFECTIEUSES Membres du Jury de la Thèse : Dr. Patricia RENESTO Rapporteur Pr. Max MAURIN Rapporteur Dr. Florence FENOLLAR Membre du Jury Pr. Pierre ͲEdouard FOURNIER Directeur de thèse Unité de Recherche sur les Maladies Infectieuses et Tropicales Émergentes UM63, CNRS 7278, IRD 198, Inserm 1095 Avant Propos Le format de présentation de cette thèse correspond à une recommandation de la spécialité Maladies Infectieuses et Microbiologie, à l’intérieur du Master de Sciences de la Vie et de la Santé qui dépend de l’Ecole Doctorale des Sciences de la Vie de Marseille. Le candidat est amené àrespecter des règles qui lui sont imposées et qui comportent un format de thèse utilisé dans le Nord de l’Europe permettant un meilleur rangement que les thèses traditionnelles. Par ailleurs, la partie introduction et bibliographie est remplacée par une revue envoyée dans un journal afin de permettre une évaluation extérieure de la qualité de la revue et de permettre àl’étudiant de le commencer le plus tôt possible une bibliographie exhaustive sur le domaine de cette thèse. Par ailleurs, la thèse est présentée sur article publié, accepté ou soumis associé d’un bref commentaire donnant le sens général du travail. -

IAP Guidelines on Rickettsial Diseases in Children
G U I D E L I N E S IAP Guidelines on Rickettsial Diseases in Children NARENDRA RATHI, *ATUL KULKARNI AND #VIJAY Y EWALE; FOR INDIAN A CADEMY OF PEDIATRICS GUIDELINES ON RICKETTSIAL DISEASES IN CHILDREN COMMITTEE From Smile Healthcare, Rehabilitation and Research Foundation, Smile Institute of Child Health, Ramdaspeth, Akola; *Department of Pediatrics, Ashwini Medical College, Solapur; and #Dr Yewale Multispeciality Hospital for Children, Navi Mumbai; for Indian Academy of Pediatrics “Guidelines on Rickettsial Diseases in Children” Committee. Correspondence to: Dr Narendra Rathi, Consultant Pediatrician, Smile Healthcare, Rehabilitation & Research Foundation, Smile Institute of Child Health, Ramdaspeth, Akola, Maharashtra, India. [email protected]. Objective: To formulate practice guidelines on rickettsial diseases in children for pediatricians across India. Justification: Rickettsial diseases are increasingly being reported from various parts of India. Due to low index of suspicion, nonspecific clinical features in early course of disease, and absence of easily available, sensitive and specific diagnostic tests, these infections are difficult to diagnose. With timely diagnosis, therapy is easy, affordable and often successful. On the other hand, in endemic areas, where healthcare workers have high index of suspicion for these infections, there is rampant and irrational use of doxycycline as a therapeutic trial in patients of undifferentiated fevers. Thus, there is a need to formulate practice guidelines regarding rickettsial diseases in children in Indian context. Process: A committee was formed for preparing guidelines on rickettsial diseases in children in June 2016. A meeting of consultative committee was held in IAP office, Mumbai and scientific content was discussed. Methodology and results were scrutinized by all members and consensus was reached.