Medical Parasitology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diagnostic Code Descriptions (ICD9)
INFECTIONS AND PARASITIC DISEASES INTESTINAL AND INFECTIOUS DISEASES (001 – 009.3) 001 CHOLERA 001.0 DUE TO VIBRIO CHOLERAE 001.1 DUE TO VIBRIO CHOLERAE EL TOR 001.9 UNSPECIFIED 002 TYPHOID AND PARATYPHOID FEVERS 002.0 TYPHOID FEVER 002.1 PARATYPHOID FEVER 'A' 002.2 PARATYPHOID FEVER 'B' 002.3 PARATYPHOID FEVER 'C' 002.9 PARATYPHOID FEVER, UNSPECIFIED 003 OTHER SALMONELLA INFECTIONS 003.0 SALMONELLA GASTROENTERITIS 003.1 SALMONELLA SEPTICAEMIA 003.2 LOCALIZED SALMONELLA INFECTIONS 003.8 OTHER 003.9 UNSPECIFIED 004 SHIGELLOSIS 004.0 SHIGELLA DYSENTERIAE 004.1 SHIGELLA FLEXNERI 004.2 SHIGELLA BOYDII 004.3 SHIGELLA SONNEI 004.8 OTHER 004.9 UNSPECIFIED 005 OTHER FOOD POISONING (BACTERIAL) 005.0 STAPHYLOCOCCAL FOOD POISONING 005.1 BOTULISM 005.2 FOOD POISONING DUE TO CLOSTRIDIUM PERFRINGENS (CL.WELCHII) 005.3 FOOD POISONING DUE TO OTHER CLOSTRIDIA 005.4 FOOD POISONING DUE TO VIBRIO PARAHAEMOLYTICUS 005.8 OTHER BACTERIAL FOOD POISONING 005.9 FOOD POISONING, UNSPECIFIED 006 AMOEBIASIS 006.0 ACUTE AMOEBIC DYSENTERY WITHOUT MENTION OF ABSCESS 006.1 CHRONIC INTESTINAL AMOEBIASIS WITHOUT MENTION OF ABSCESS 006.2 AMOEBIC NONDYSENTERIC COLITIS 006.3 AMOEBIC LIVER ABSCESS 006.4 AMOEBIC LUNG ABSCESS 006.5 AMOEBIC BRAIN ABSCESS 006.6 AMOEBIC SKIN ULCERATION 006.8 AMOEBIC INFECTION OF OTHER SITES 006.9 AMOEBIASIS, UNSPECIFIED 007 OTHER PROTOZOAL INTESTINAL DISEASES 007.0 BALANTIDIASIS 007.1 GIARDIASIS 007.2 COCCIDIOSIS 007.3 INTESTINAL TRICHOMONIASIS 007.8 OTHER PROTOZOAL INTESTINAL DISEASES 007.9 UNSPECIFIED 008 INTESTINAL INFECTIONS DUE TO OTHER ORGANISMS -

A Screening-Based Approach to Circumvent Tumor Microenvironment
JBXXXX10.1177/1087057113501081Journal of Biomolecular ScreeningSingh et al. 501081research-article2013 Original Research Journal of Biomolecular Screening 2014, Vol 19(1) 158 –167 A Screening-Based Approach to © 2013 Society for Laboratory Automation and Screening DOI: 10.1177/1087057113501081 Circumvent Tumor Microenvironment- jbx.sagepub.com Driven Intrinsic Resistance to BCR-ABL+ Inhibitors in Ph+ Acute Lymphoblastic Leukemia Harpreet Singh1,2, Anang A. Shelat3, Amandeep Singh4, Nidal Boulos1, Richard T. Williams1,2*, and R. Kiplin Guy2,3 Abstract Signaling by the BCR-ABL fusion kinase drives Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL) and chronic myelogenous leukemia (CML). Despite their clinical activity in many patients with CML, the BCR-ABL kinase inhibitors (BCR-ABL-KIs) imatinib, dasatinib, and nilotinib provide only transient leukemia reduction in patients with Ph+ ALL. While host-derived growth factors in the leukemia microenvironment have been invoked to explain this drug resistance, their relative contribution remains uncertain. Using genetically defined murine Ph+ ALL cells, we identified interleukin 7 (IL-7) as the dominant host factor that attenuates response to BCR-ABL-KIs. To identify potential combination drugs that could overcome this IL-7–dependent BCR-ABL-KI–resistant phenotype, we screened a small-molecule library including Food and Drug Administration–approved drugs. Among the validated hits, the well-tolerated antimalarial drug dihydroartemisinin (DHA) displayed potent activity in vitro and modest in vivo monotherapy activity against engineered murine BCR-ABL-KI–resistant Ph+ ALL. Strikingly, cotreatment with DHA and dasatinib in vivo strongly reduced primary leukemia burden and improved long-term survival in a murine model that faithfully captures the BCR-ABL-KI–resistant phenotype of human Ph+ ALL. -

Molecular Detection of Human Parasitic Pathogens
MOLECULAR DETECTION OF HUMAN PARASITIC PATHOGENS MOLECULAR DETECTION OF HUMAN PARASITIC PATHOGENS EDITED BY DONGYOU LIU Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2013 by Taylor & Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Version Date: 20120608 International Standard Book Number-13: 978-1-4398-1243-3 (eBook - PDF) This book contains information obtained from authentic and highly regarded sources. Reasonable efforts have been made to publish reliable data and information, but the author and publisher cannot assume responsibility for the validity of all materials or the consequences of their use. The authors and publishers have attempted to trace the copyright holders of all material reproduced in this publication and apologize to copyright holders if permission to publish in this form has not been obtained. If any copyright material has not been acknowledged please write and let us know so we may rectify in any future reprint. Except as permitted under U.S. Copyright Law, no part of this book may be reprinted, reproduced, transmitted, or utilized in any form by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying, microfilming, and recording, or in any information storage or retrieval system, without written permission from the publishers. For permission to photocopy or use material electronically from this work, please access www.copyright.com (http://www.copyright.com/) or contact the Copyright Clearance Center, Inc. -

The Alkaloid Emetine As a Promising Agent for the Induction and Enhancement of Drug-Induced Apoptosis in Leukemia Cells
737-744 26/7/07 08:26 Page 737 ONCOLOGY REPORTS 18: 737-744, 2007 737 The alkaloid emetine as a promising agent for the induction and enhancement of drug-induced apoptosis in leukemia cells MAREN MÖLLER1, KERSTIN HERZER3, TILL WENGER2,4, INGRID HERR2 and MICHAEL WINK1 1Institute of Pharmacy and Molecular Biotechnology, University of Heidelberg, Im Neuenheimer Feld 364; 2Molecular OncoSurgery, Department of Surgery, University and German Cancer Research Center, Im Neuenheimer Feld 365, 69120 Heidelberg; 31st Department of Internal Medicine, University of Mainz, Langenbeckstrasse 1, 55131 Mainz, Germany; 4Centre d'Immunologie de Marseille-Luminy, Marseille, France Received January 8, 2007; Accepted February 20, 2007 Abstract. Emetine, a natural alkaloid from Psychotria caused mainly by two isoquinoline alkaloids, emetine and ipecacuanha, has been used in phytomedicine to induce cephaeline, having identical effects regarding the irritation of vomiting, and to treat cough and severe amoebiasis. Certain the respiratory tract (1). Nowadays, ipecac syrup is no longer data suggest the induction of apoptosis by emetine in recommended for the routine use in the management of leukemia cells. Therefore, we examined the suitability of poisoned patients (2) and recently a guideline on the use of emetine for the sensitisation of leukemia cells to apoptosis ipecac syrup was published, stating that ‘the circumstances in induced by cisplatin. In response to emetine, we found a which ipecac-induced emesis is the appropriate or desired strong reduction in viability, an induction of apoptosis and method of gastric decontamination are rare’ (3). Moreover, at caspase activity comparable to the cytotoxic effect of present there is a demand to remove ipecac from the over- cisplatin. -
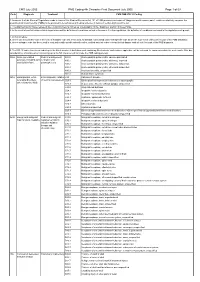
CMS PMB ICD-10 Coding
CMS July 2005 PMB Coding 4th Character Final Dcoument July 2005 Page 1 of 69 Code Diagnosis Treatment CMS PMB ICD-10 Coding 1. Annexure A of the General Regulations made in terms of the Medical Schemes Act, 131 of 1998 provides a schedule of “diagnosis and treatment pairs”, which cumulatively comprise the prescribed minimum benefits (PMBs) to be provided to beneficiaries of medical schemes in terms of section 29(1)(o) of the Act. 2. The attached ICD10 codes represent the Council for Medical Schemes’ interpretation of the “diagnosis” portion of these PMBs. 3. In the event of conflict between this interpretation and the definition of conditions set out in Annexure A to the regulations, the definition of conditions contained in the regulations will prevail. 4. In this schedule: a. where only the primary code in the form of a dagger code has been used, all asterisk codes listed under that specific code as per the ICD-10 set of books form part of the PMB diagnosis; b. where a dagger code has been used in conjunction with specific asterisk codes, omitted asterisk codes relevant to that dagger code do not form part of the PMB diagnosis. 5. The ICD-10 codes have been coded up to the 4th character. A draft document containing 5th character codes where applicable, will be released for comments within the next month. After due consideration, a final document containing up to the 5th character will conclude the PMB coding process. 906A Acute generalised Medical management; A80.0 Acute paralytic poliomyelitis, vaccine-associated paralysis, including -

2011/109440 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date _ . 9 September 2011 (09.09.2011) 2011/109440 Al (51) International Patent Classification: [CH/CH]; Chemin Des Chevreuils 1, 1188 Gimel (CH). C12Q 1/68 (2006.01) G01N 33/53 (2006.01) HOLTERMAN, Daniel [US/US]; 14465 North 14th St., Phoenix, AZ 85022 (US). (21) International Application Number: PCT/US201 1/026750 (74) Agent: AKHAVAN, Ramin; Caris Life Sciences, Inc., 6655 N. MacArthur Blvd., Irving, TX 75039 (US). (22) International Filing Date: 1 March 201 1 (01 .03.201 1) (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, English (25) Filing Language: AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (26) Publication Language: English CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (30) Priority Data: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 61/274,124 1 March 2010 (01 .03.2010) US KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 61/357,5 17 22 June 2010 (22.06.2010) US ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, 61/364,785 15 July 2010 (15.07.2010) us NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (71) Applicant (for all designated States except US): CARIS SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, LIFE SCIENCES LUXEMBOURG HOLDINGS [LU/ TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Cutaneous Amebiasis in Pediatrics
OBSERVATION Cutaneous Amebiasis in Pediatrics Mario L. Magan˜a, MD; Jorge Ferna´ndez-Dı´ez, MD; Mario Magan˜a,MD Background: Cutaneous amebiasis (CA), which is still Conclusions: Cutaneous amebiasis always presents with a health problem in developing countries, is important painful ulcers. The ulcers are laden with amebae, which to diagnose based on its clinical and histopathologic are relatively easy to see microscopically with routine features. stains. Erythrophagocytosis is an unequivocal sign of CA. Amebae reach the skin via 2 mechanisms: direct and in- Observations: Retrospective medical record review of direct. Amebae are able to reach the skin if there is a lac- 26 patients with CA (22 adults and 4 children) treated from eration (port of entry) and if conditions in the patient 1955 to 2005 was performed. In addition to the age and are favorable. Amebae are able to destroy tissues by means sex of the patients, the case presentation, associated ill- of their physical activity, phagocytosis, enzymes, secre- ness or factors, and method of establishing the diagnosis, tagogues, and other molecules. clinical pictures and microscopic slides were also analyzed. Arch Dermatol. 2008;144(10):1369-1372 UTANEOUS AMEBIASIS (CA) ria, are opportunistic organisms that act as can be defined as damage pathogens, usually in the immunocompro- to the skin and underly- mised host, who can develop disease in any ing soft tissues by tropho- organ, such as the skin and central ner- zoites of Entamoeba histo- vous system. This kind of amebiasis has be- lytica, the only pathogenic form for humans. come more common during the last few C 8-18 Cutaneous amebiasis may be the only ex- years. -

ED227273.Pdf
DOCUMENT RESUft ED 227 273 y CE 035 300 ' TITLE APharmacy Spicialist, Militkry Curriculum Materials for Vocation47 andileChlaical Education. INSTITuTION Air Force Training Command, Sheppa* AFB, Tex.; Ohio State Univ., Columbus. Natfonal Center for Research in Vocational Education. SPONS AGENCY Office of Education (DHEW)x Washington, D.C. PUB DATE 18 Jul 75 NOTE 774p.; Some pages are marginally legible. ,PUB TYPE Guides - Classroom Use Guides (For Teachers) (052), , EDRS ?RICE 14P05/PC31 Plus Postage. ` DESCRIPTORS Behavioral Objectives; Course Descriptions; A Curriculum Guides; Drug Abuse; Drug,Therapy; 4Drug Use; Learning Activities; Lesson Plans; *Pharmaceutical Education; Pharmacists; *Pharmacology; *Pharmacy; Postsecondary Education; Programed Instructional Materials; Textbooks; Workbooks IDENTIFIERS. Military CuFr.iculum Project liBSTRACT These teacher and studdnt,materials for a . postsecondary-level course in pharmacy comprise one of a numberof military-developed curriculum packages selected for adaptation to voCational instruction 'and curriculum dei7elopment in acivilian setting. The purpose stated for the 256-hour course iS totrain students in the basic technical phases of pharmacy and theminimum essential knowledge and skills necessaryior.the compounding and - dispensing of drugs, the economical operation of a pharmacy,and the proper use of drugs, chemicals, andbiological products. The course consists of three blocks of instruction. Block I contains four, lessons: pharmaceutical calculations I and laboratory,inorganic chemistry, and organic chemistry. The five lessons in Block II cover anatomy ,and physiology, introduction topharmacoloe, toxicology, drug abuse, and pharmaceutical and medicinal agents. Block III provides five lessons: phdrmaceutical calculations\I and II, techniques"of pharmaceutical compounding, pharmaceutiCal dosage for s, and compounding laboratbry. Instructormaterials include a cb se chart, lesson plans, and aplan of instruction detailing instructional,bnits, criterion objectives, lesson duration,and support materials needed. -

Pharmacy Data Management Drug Exception List
Pharmacy Data Management Drug Exception List Patch PSS*1*127 updated the following drugs with the listed NCPDP Multiplier and NCPDP Dispense Unit. These two fields were added as part of this patch to the DRUG file (#50). Please refer to the Release notes for ePharmacy/ECME Enhancements for Pharmacy Release Notes (BPS_1_5_EPHARMACY_RN_0907.PDF) on the VistA Documentation Library (VDL). The IEN column reflects the IEN for the VA PRODUCT file (#50.68). The ePharmacy Change Control Board provided the following list of drugs with the specified NCPDP Multiplier and NCPDP Dispense Unit values. This listing was used to update the DRUG file (#50) with a post install routine in the PSS*1*127 patch. NCPDP File 50.68 NCPDP Dispense IEN Product Name Multiplier Unit 2 ATROPINE SO4 0.4MG/ML INJ 1.00 ML 3 ATROPINE SO4 1% OINT,OPH 3.50 GM 6 ATROPINE SO4 1% SOLN,OPH 1.00 ML 7 ATROPINE SO4 0.5% OINT,OPH 3.50 GM 8 ATROPINE SO4 0.5% SOLN,OPH 1.00 ML 9 ATROPINE SO4 3% SOLN,OPH 1.00 ML 10 ATROPINE SO4 2% SOLN,OPH 1.00 ML 11 ATROPINE SO4 0.1MG/ML INJ 1.00 ML 12 ATROPINE SO4 0.05MG/ML INJ 1.00 ML 13 ATROPINE SO4 0.4MG/0.5ML INJ 1.00 ML 14 ATROPINE SO4 0.5MG/ML INJ 1.00 ML 15 ATROPINE SO4 1MG/ML INJ 1.00 ML 16 ATROPINE SO4 2MG/ML INJ 1.00 ML 18 ATROPINE SO4 2MG/0.7ML INJ 0.70 ML 21 ATROPINE SO4 0.3MG/ML INJ 1.00 ML 22 ATROPINE SO4 0.8MG/ML INJ 1.00 ML 23 ATROPINE SO4 0.1MG/ML INJ,SYRINGE,5ML 5.00 ML 24 ATROPINE SO4 0.1MG/ML INJ,SYRINGE,10ML 10.00 ML 25 ATROPINE SO4 1MG/ML INJ,AMP,1ML 1.00 ML 26 ATROPINE SO4 0.2MG/0.5ML INJ,AMP,0.5ML 0.50 ML 30 CODEINE PO4 30MG/ML -

CNS Infections: LP Is an Important Test in the Diagnosis of Meningitis and Encephalitis
Bacterial meningitis: bacterial infection of the Leptomeninges. • The most common cause of bacterial meningitis in neonates is B group streptococcus and Gram negative bacteria (E.coli and Citrobacter)-ampicillin and gentamycin or third generation cephalosporines. Citrobacter is associated with high incidence of multiple brain abscesses. The most common cause of bacterial meningitis in age group 3 months-5 years are H.influenzae and streptococcus pneumoniae-penecillinnes and third generation cephalosporines. The most common cause in older children and adults are strept. Pneumoniae and Neisseria meningitides-penecillinnes and cephalosporines. The most common cause of viral meningitis is enteroviruses (echo and Cocksakie viruses). • The clinical picture depends on the age: neonates and children present with sepsis. Adults and old children (fever, headaches, vomiting, neck stiffness and decreased level of consciousness. Hemorrhagic rash in case of Neisseria meningitides). Patients should be started on high dose Abs and in the absence of increased ICP , LP should be performed (high protein, decreased glucose, pleocytosis. Neutrophils in thousands. In the presence of raised ICP start Abs and get CT head. Duration of treatment 10-14 days iv Abs. • Individuals exposed to N.meningidites infection should be given Rifampicin for 3-5 days. Vaccines are available. • Talk about TB meningitis (basal meningitis with multiple cranial nerve palsies and sometimes hydrocephalus) and • Discuss the indications and contraindications of LP in CNS infections: LP is an important test in the diagnosis of meningitis and encephalitis. It is contraindicated in the presence signs of increased ICP due to space occupying lesion such as abscess, subdural and epidural empyema (papilloedema, decreased level of consciousness). -

Drug Resistance in the Sexually Transmitted Protozoan Trichomonas Vaginalis
Cell Research (2003); 13(4):239-249 http://www.cell-research.com Drug resistance in the sexually transmitted protozoan Trichomonas vaginalis 1, 2 1 1 2 REBECCA L DUNNE , LINDA A DUNN , PETER UPCROFT , PETER J O'DONOGHUE , JACQUELINE A UPCROFT1,* 1 The Queensland Institute of Medical Research; The Australian Centre for International and TropicalHealth and Nutrition; Brisbane, Queensland 4029, Australia 2 The School of Molecular and Microbial Science, University of Queensland, Brisbane, Queensland 4072, Australia ABSTRACT Trichomoniasis is the most common, sexually transmitted infection. It is caused by the flagellated protozoan parasite Trichomonas vaginalis. Symptoms include vaginitis and infections have been associated with preterm delivery, low birth weight and increased infant mortality, as well as predisposing to HIV/AIDS and cervical cancer. Trichomoniasis has the highest prevalence and incidence of any sexually transmitted infection. The 5- nitroimidazole drugs, of which metronidazole is the most prescribed, are the only approved, effective drugs to treat trichomoniasis. Resistance against metronidazole is frequently reported and cross-resistance among the family of 5-nitroimidazole drugs is common, leaving no alternative for treatment, with some cases remaining unresolved. The mechanism of metronidazole resistance in T. vaginalis from treatment failures is not well understood, unlike resistance which is developed in the laboratory under increasing metronidazole pressure. In the latter situation, hydrogenosomal function which is involved in activation of the prodrug, metronidazole, is down-regulated. Reversion to sensitivity is incomplete after removal of drug pressure in the highly resistant parasites while clinically resistant strains, so far analysed, maintain their resistance levels in the absence of drug pressure. -

Final1-Aritmo-Final-Report-V2-0Final.Pdf
ARITMO Final Report PROJECT FINAL REPORT Grant Agreement number: 241679 Project acronym: ARITMO Project title: Arrhythmogenic potential of drugs Funding Scheme: Small or Medium-Scale Focused Research Project Period covered: from 1st January 2010 to 30th June 2013 Name of the scientific representative of the project's co-ordinator, Title and Organisation: Prof. Miriam CJM Sturkenboom, Erasmus Universitair Medisch Centrum Rotterdam Tel: +31 10 704 4126 Fax: +31 10 704 4722 E-mail: [email protected] Project website1 address: www.aritmo-project.org 1 The home page of the website should contain the generic European flag and the FP7 logo which are available in electronic format at the Europa website (logo of the European flag: http://europa.eu/abc/symbols/emblem/index_en.htm ; logo of the 7th FP: http://ec.europa.eu/research/fp7/index_en.cfm?pg=logos). The area of activity of the project should also be mentioned. © Copyright 2013 ARITMO Consortium 1 ARITMO Final Report Table of contents Table of contents ................................................................................................................................................................. 2 1. Final publishable summary report ................................................................................................................................ 3 1.1 Executive summary ................................................................................................................................................. 3 1.2 Description of project context and